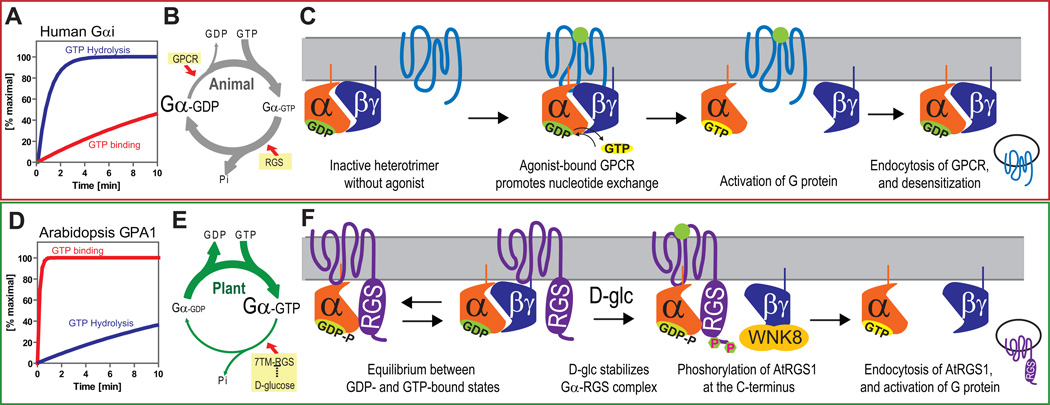
Figure 7. Model of sustained G protein activation in Arabidopsis; comparison of activation mechanisms between fast and slow nucleotide exchanging G proteins.
(A, D) Rate of guanine nucleotide exchange of human G protein is slower than that of GTP hydrolysis. However, Arabidopsis AtGPA1 rapidly releases GDP, while GTP hydrolysis is slow. (B, E) Based on these intrinsic properties, human G proteins require GPCRs to form the active GTP-bound state. In contrast, AtGPA1 requires a constitutively active 7TM-RGS protein, AtRGS1, to keep the inactive GDP-bound state. Genetic evidence suggests that D-glucose inhibits AtRGS1 to activate the Arabidopsis G protein pathway. (C, F) Many human GPCRs are endocytosed after phosphorylation and subsequent endocytosis causes desensitization of G protein signaling. In Arabidopsis, in the absence of glucose, Gα subunit binding is in equilibrium between the RGS and Gβ dimerization interfaces, both shared on the Gα subunit. Glucose shifts the equilibrium toward the Gα-RGS dimer increasing the time the Gβγ remains free from the heterotrimer. The free Gβγ dimer recruits AtWNK8 to phosphorylate AtRGS1 at its C terminus. Phosphorylation is requisite for AtRGS1 endocytosis. Endocytosis causes uncoupling of AtGPA1 from its inhibitor, AtRGS1 and subsequent sustained activation of G protein signaling.