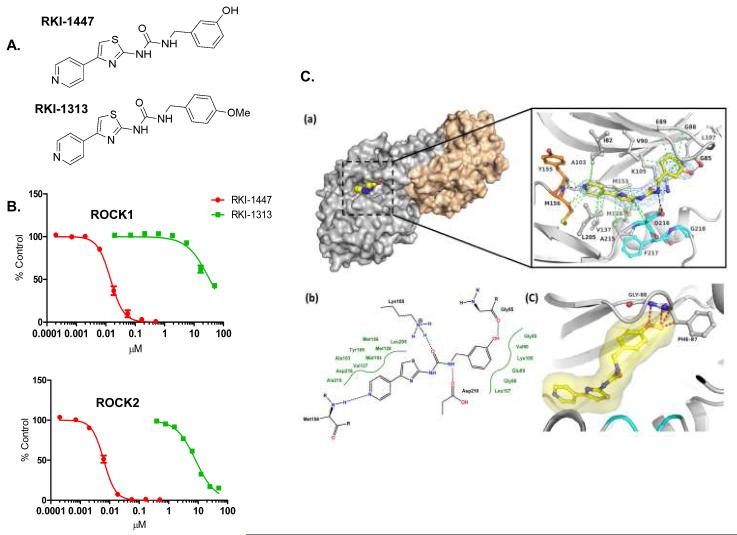
Figure 1. RKI-1447 and RKI-1313 Chemical structures, in vitro ROCK inhibitory activity and RKI-1447-ROCK1 co-crystal structure.
1A. Chemical structure of RKI-1447 and RKI-1313. 1B. RKI-1447 is much more potent than RKI-1313 at inhibiting both ROCK1 and ROCK2. 1C. Structure of the RKI-1447-ROCK1 complex. (a) Surface presentation of one of the two ROCK1 dimers in complex with RKI-1447 determined by X-ray crystallography at 2.9 Å resolution and detailed binding interactions in the ATP site. The hinge region is indicated in orange, the DFG motif in cyan, and RKI-1447 in yellow. Displayed in blue is the 2 Fo-Fc electron density, contoured at 1σ around the RKI-1447 inhibitor. The Fo-Fc electron density map with the RKI-1447 inhibitor omitted during refinement is shown in the supplementary data (Supplementary Figure S4). The hydrogen bonding and van der Waal interactions are shown as black and green dotted lines, respectively. (b) Schematic presentation of the binding interactions between RKI-1447 and the ATP site. (c) Model of RKI-1313 bound to the binding site of RKI-1447.