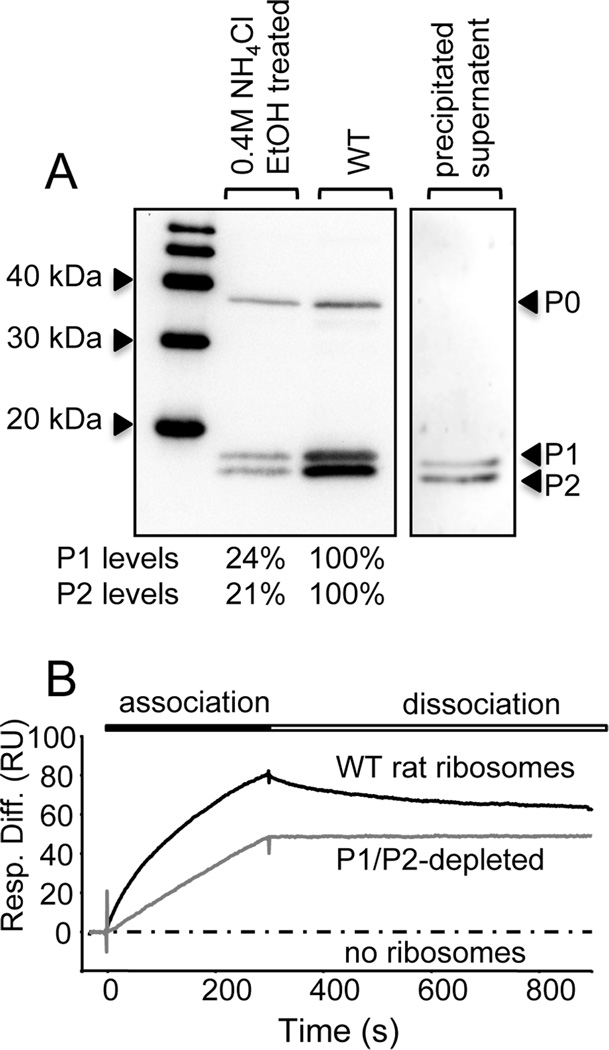
Fig. 4.
Depletion of P1 and P2 proteins from rat ribosomes and its effect on ribosome binding by RTA. (A) Ribosomes were treated with NH4Cl (0.4M) followed by the addition of ethanol (50% [v/v]) to selectively remove P1and P2. Following high speed centrifugation, the ribosomal pellet and the supernatant containing stripped P1/P2 proteins were collected and analyzed by western blotting with a monoclonal antibody against the conserved C-termini of P-proteins. The indicated P1/P2 protein levels in depleted ribosomes are expressed as a percentage the P1/P2 protein levels in untreated samples, normalized to P0 levels. (B) The interaction of RTA with WT and P1/P2-depleted rat ribosomes measured by surface plasmon resonance. N-terminal His-tagged RTA was coupled to an NTA chip on the Biacore 3000 and ribosomes (5 nM) were passed over the surface for 5 min and dissociation was monitored for 10 min. The interaction of ribosomes with a control ligand (EGFP) was analyzed in parallel and subtracted from the signal obtained for the target surface (RTA), to account for non-specific binding.