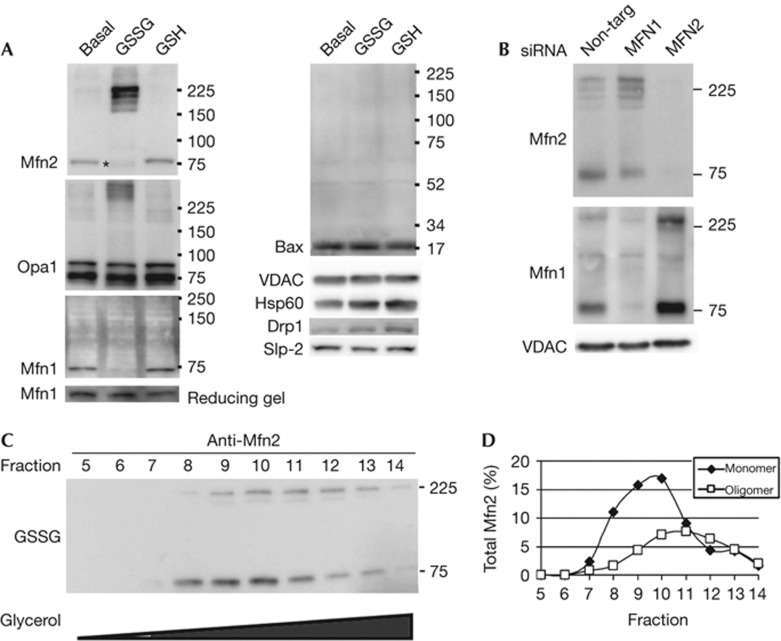
Figure 2.
Electrophoretic mobility of the fusion machinery is altered on treatment with GSSG. (A) Western blots from samples run under non-reducing conditions with proteins isolated from mitochondria incubated under basal fusion conditions either untreated, or treated with 0.5 mM GSSG or 0.5 mM GSH. Asterisk indicates the more rapid migration of the Mfn2 monomer with GSSG treatment. (B) Mitochondria were isolated from cells treated with control siRNA, Mfn1 siRNA, or Mfn2 siRNA. Following the basal fusion reaction in the presence of 0.5 mM GSSG, proteins were isolated and analysed by western blotting from a gel run under non-reducing conditions. The membrane was reduced in buffer containing β-mercaptoethanol prior immunoblotting with Mfn1, allowing the reduced form of the protein to be recognized. Western blots are representative of at least three independent experiments. (C) Proteins from digitonin-permeabilized GSSG-treated mitochondria were separated by a 5–24% glycerol gradient. Fractions 5–14 (9–18%) were analysed under non-denaturing conditions and probed for Mfn2. (D) Quantification of the monomeric and oligomeric Mfn2 bands. The oligomeric form migrates further into the gradient than the monomeric form, indicating they are larger protein complexes. GSH, reduced glutathione; GSSG, oxidized glutathione; Mfn1, Mitofusin 1; Mfn2, Mitofusin 2; Opa1, Optical Atrophy 1; siRNA, short interfering RNA.