FIGURE 2. In vitro DNA-binding activities of WT and T127L/S128I CRP proteins.
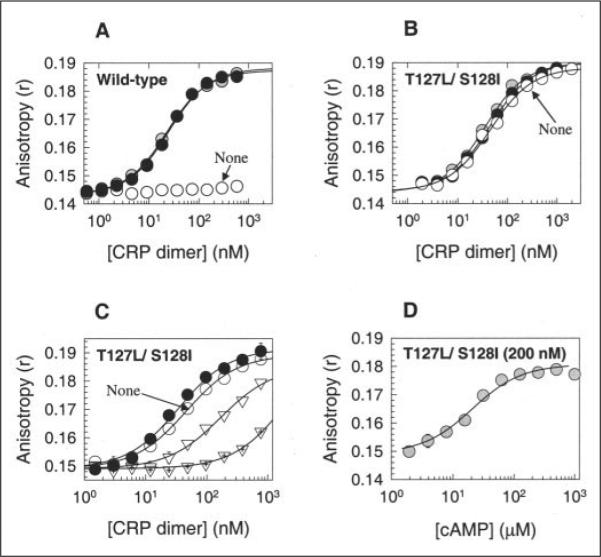
A and B, for WT CRP and T127L/S128I CRP, respectively, the activities were measured by the fluorescence polarization method. Open circles, no cAMP; shaded circles, 0.1 mM cAMP; closed circles, 1 mM cAMP. Solid lines show the best fit of the data to an equation described by Lundblad et al. (30). C, cGMP caused the loss of DNA-binding activity in T127L/S128I CRP, and cAMP restored the activity. Open circles, no cAMP; inverted open triangles, 0.1 mM cGMP; crossed inverted open triangles, 1 mM cGMP; closed circles, 1 mM cGMP + 1 mM cAMP. D, T127L/S128I CRP (200 nM) showed better affinity for cAMP than for cGMP. The DNA-binding activity of the protein was measured with various cAMP levels in the presence of 1 mM cGMP. Both WT and T127L/S128I CRP proteins were His6-tagged at their C termini.
