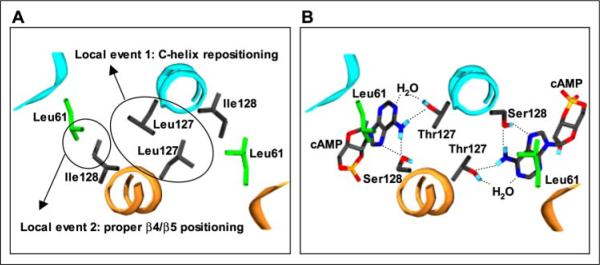
FIGURE 5. Structural model for high constitutive activity of T127L/S128I CRP.
The hypothesized local structure of T127L/S128I CRP (A) was derived from the structure of active cAMP-bound WT CRP (B) (Protein Data Bank code 1CGP). In this view along a portion of the C-helices, the DNA-binding domains are in the distance. The T127L/S128I CRP structure is not meant to represent the actual one, but rather to indicate a reasonable set of Leu127 and Ile128 conformers that might explain its high constitutive activity. In each figure, two subunits are differently colored. In A, the side chains of Leu61, Leu127, and Ile128 residues are shown. In B, the side chains of Leu61, Thr127, and Ser128 residues and cAMP molecules are shown, and the known interactions (adopted from Ref. 14) are also indicated. The structure was generated using Swiss-PdbViewer Version 3.7.