Abstract
Endogenous stem cell recruitment to the site of skeletal injury is key to enhanced osseous remodeling and neovascularization. To this end, this study utilized a novel bone allograft coating of poly(lactic-co-glycolic acid) (PLAGA) to sustain the release of FTY720, a selective agonist for sphingosine 1-phosphate (S1P) receptors, from calvarial allografts. Uncoated allografts, vehicle-coated, low dose FTY720 in PLAGA (1:200 w:w) and high dose FTY720 in PLAGA (1:40) were implanted into critical size calvarial bone defects. The ability of local FTY720 delivery to promote angiogenesis, maximize osteoinductivity and improve allograft incorporation by recruitment of bone progenitor cells from surrounding soft tissues and microcirculation was evaluated. FTY720 bioactivity after encapsulation and release was confirmed with sphingosine kinase 2 assays. HPLC-MS quantified about 50% loaded FTY720 release of the total encapsulated drug (4.5 µg) after 5 days. Following 2 weeks of defect healing, FTY720 delivery led to statistically significant increases in bone volumes compared to controls, with total bone volume increases for uncoated, coated, low FTY720 and high FTY720 of 5.98, 3.38, 7.2 and 8.9 mm3, respectively. The rate and extent of enhanced bone growth persisted through week 4 but, by week 8, increases in bone formation in FTY720 groups were no longer statistically significant. However, micro-computed tomography (microCT) of contrast enhanced vascular ingrowth (MICROFIL®) and histological analysis showed enhanced integration as well as directed bone growth in both high and low dose FTY720 groups compared to controls.
Keywords: Bone tissue engineering, Drug delivery, Angiogenesis, Osseointegration, Massive Allograft
Introduction
Bone graft procedures number over 500,000 annually in the United States, with allograft tissue used for 33% of the bone graft operations in 1996 (Boyce et al. 1999). To address the growing clinical demand for effective bone reconstruction materials, bone allograft substitutes (allografts) from cadavers and donors have been utilized extensively to repair skeletal defects. Though cancellous bone autograft transplants are the standard for bone reconstruction (Giannoudis et al. 2005), the graft mass is inadequate for massive bone defects and bone harvesting leads to significant donor site morbidity. Allografts avoid these problems and their biocompatibility and osteoconductivity are superior to synthetic bone substitute materials (Delloye et al. 2007). However, poor remodeling and lack of vascularization of the processed bone allograft can lead to failure from recurring infection, fracture, or nonunions (Delloye et al. 2007; Thompson et al. 1993; Enneking and Campanacci 2001; Mankin et al. 2005). Complications associated with cadaver allograft occur 30–60% of the time for massive transplants (Delloye et al. 1988) and allografts lose 50% strength after 10 years in vivo (Wheeler and Enneking 2005). Moreover, recent systematic evaluations of bone substitutes in critical size cranial defects confirm that the performance of autologous bone grafts is far superior to that of various other bone substitutes (Mokbel et al. 2008). Cell augmentation, graft processing and use of bone morphogenetic proteins (BMPs) have been explored as possible means to increase allograft incorporation in the defect site. (Johnson and Urist 2000) showed that hBMP in an allogeneic, autolysed antigen-free bone allograft provided a good structural and delivery system for inducing bone formation by causing internal remodeling following femoral reconstruction after failed fracture healing. However, they supplemented all defects greater than 2 cm with an autogenic bone graft. BMP-2’s relatively short elimination half-life of 5.6 days while locally administered in a collagen carrier (Sellers et al. 2000) has been a major drawback when used to coat off-the-shelf acellular constructs like allografts because the limited bone remodeling in a critical size defect occurs over months. One recent approach to counteract this limitation has been to use allografts coated with freeze-dried self-complimentary AAV-2.5 BMP-2 adeno-associated virus (Yazici et al. 2011). Local, sustained physiologic levels of BMP-2 resulted in higher strength and bone volume compared to both allograft and autograft treatments. However, the simplicity of small molecules compared to biologic therapies make pursuing alternative treatments worthwhile.
It is well established that allograft vascularization and new bone formation are critical for allograft survival and incorporation. Significant interest has emerged in the development of technologies to enhance the integration and efficacy of processed cadaver allografts used for bone reconstruction by promoting rapid vascularization upon implantation, as well as by encouraging the recruitment of endogenous host stem and progenitor cells. Several factors such as the presence or absence of living, histocompatible, committed bone-forming cells (Stevenson et al. 1996), embryological mode of development and the quantity of cancellous bone (Pinholt et al. 1994) have been suggested to have an influence on the vascularization and hence engraftment of the bone grafts. This work continues to investigate the effects of local, sustained delivery of sphingosine 1-phosphaste (S1P) receptor targeted drugs to induce neovascularization and improve healing outcomes in tissue engineering. S1P is an autocrine and paracrine signaling small molecule that is known to affect proliferation, survival and migration of endothelial cells, mural cells (i.e. vascular smooth muscle cells and pericytes), osteoblasts and osteoblastic precursors through a family of high-affinity G protein-coupled receptors (S1P1–5) (Zhang et al. 1991; Cuvillier et al. 1996; Lee et al. 2001; Ryu et al. 2006; Pebay et al. 2007). S1P has also been shown to enhance angiogenesis and vascularization in various pre-clinical models in addition to regulating B and T cell trafficking and the egress of immune cells from lymph nodes (Schwab et al. 2005; Pappu et al. 2007).
In previous work, it was shown that sustained release of S1P and FTY720, an S1P1 and S1P3 agonist, from biodegradable poly(DL-lactic-co-glycolic acid) (PLAGA) promotes microvascular network growth and arteriolar expansion (Sefcik et al. 2011). It was also shown that the local delivery of FTY720 from PLAGA film promotes local microvasculature formation and cranial defect healing in a rat model (Petrie Aronin et al. 2010a). Superior osseous integration across the host-graft interface, enhancement of mechanical properties, increased smooth muscle cell investment and reduction in leukocyte recruitment was also evident in FTY720-treated groups in a rat tibial defect model (Petrie Aronin et al. 2010b). FTY720, a small molecule drug that modulates S1P receptors, is more stable and has a longer half-life than many growth factors that are candidates for clinical applications. FTY720 has been FDA approved as an oral drug for the treatment of multiple sclerosis (Sharma et al. 2011). FTY720 has been efficiently loaded in PLAGA microsphere scaffolds and degradable implant coatings to ensure that the drug is released for several weeks following implantation. Critical size cranial defects have been used to examine the efficacy of drugs and other potential therapies for maxillofacial nonunions (Schmitz and Hollinger 1986). However, studies with subperiosteal implants help provide initial insights into revascularization and bone regeneration qualities of the implant (Chen et al. 1994).
In this study, FTY720 coating efficacy on cranial subperiosteal implants was explored and then a semicircular calvarial allograft was used to evaluate total bone volume growth, bone remodeling at the interface, and directed bony ingrowth into the defect area. FTY720’s effect on increased vascularization, arteriogenesis and progenitor cell recruitment in the defect area was also explored.
Materials and methods
Calvarial allografts were coated with FTY720-loaded PLAGA. FTY720 encapsulation and release was quantified in vitro. To explore the potential of drug releasing allografts to integrate with the host and promote bone growth, FTY720-loaded, PLAGA-coated, or uncoated allografts were implanted in rat calvarial subperiosteum. Subsequent to the subperiosteal implant study, a semicircular allograft for the rat critical size cranial defect model was developed. The graft was placed on one side, allowing measurement of both host–graft bridging and integration and bone induction into the defect void space. FTY720 effects on total bone volume, bone growth qualities, blood vessel volume, smooth muscle cell supported blood vessels and circulating blood cells were explored.
Bone allograft harvest and coating
Parietal bone was harvested from Sprague Dawley female rat skulls (4–5 months old), trimmed to a rectangular shape (5.7 × 5.2 × 0.75 mm) and used in the subperiosteal implantation. The cranial defect study used parietal bones from 8-week-old Sprague Dawley female rats, trimmed to two semi-circles (≈5 mm diameter edge along the sagittal suture). Grafts were stripped of soft tissue, cleaned with detergent, hydrogen peroxide and ethanol sonication washes and then allowed to fully dry (DePaula et al. 2005). All bones were analyzed with micro-computed tomography (microCT) vivaCT40 scanner (SCANCO Medical, Brüttisellen, Switzerland) for volume, weighed and equally divided into treatment groups. Samples were stored at −20°C until use.
Polymer coatings used 50:50 poly(lactic-co-glycolic acid) (PLAGA; 69.85 kDa; Lakeshore Biomaterials, Birmingham, AL, USA) dissolved in dichloromethane at 1:12 (w:w). For drug loading, FTY720 (343.9 Da; Cayman Chemical, Ann Arbor, MI, USA) was agitated until dissolved in the polymer solution at either 1:200 or 1:40 of the drug to polymer (w:w). To coat allograft samples, bones were vortexed in a coating solution for 10 min. To dry the coating, bones were kept at 4°C for 24 h and then lyophilized for 24 h to extract the remaining solvent (Labconco, Kansas City, MO, USA).
FTY720 encapsulation efficiency and release
FTY720 quantities from drug-loaded, polymer-coated allografts were measured by sphingolipid extraction from the solution and HPLC-MS. For in vitro studies, FTY720 was loaded 1:200 drug: polymer (w:w) and 1:12 polymer: solvent (w:v). For encapsulation efficiency, allografts were sonicated with chloroform (1 mL) to dissolve the coating. Then, the bone was removed and methanol (3 mL) was added to the remaining solution. To measure extraction efficiency, a D-erythro-Sphingosine (C-17 base) internal standard was added (10 µL, 1 µM, Mw=285.47 Da; Avanti Polar Lipids, Alabaster, AL, USA). This mixture was sonicated for 10 min and immediately incubated at 48°C for 16 h. After cooling to room temperature, KOH (0.2 mL, 1 M) was added and the solution was centrifuged at 10,000g for 10 min at 4°C. The supernatant was collected, dried to a solid with nitrogen air-flow and stored at −20°C. Immediately prior to HPLC-MS analysis, the extraction residue was dissolved in methanol (0.3 mL) and centrifuged at 12,000g for 12 min at 4°C. Samples were analyzed with a Shimadzu UFLC High Performance Liquid Chromatograph (Columbia, MD, USA) equipped with a Supelco Discovery C18, 5 µm (125 × 2 mm) connected to an ABI 4000 QTrap triple quadrupole mass spectrometer (Applied Biosystems, USA).
For in vitro release, allografts were placed in vials containing 1 mL simulated body fluid (pH 7.2; 7.996 g NaCl, 0.35 g NaHCO3, 0.3 g KCl, 0.136 g KH2PO4, 0.095 g MgCl2, 0.278 g CaCl2, 0.06 g MgSO4 in 1 L deionized water) with 4% (w:v) fatty acid free bovine serum albumin (FAF-BSA) and maintained at 37°C with constant agitation. Each day for 5 days, the bone was moved to a new vial with fresh solution. After adding methanol (1.5 mL) and chloroform (0.5 mL) to the solution, FTY720 was extracted and quantified as described above.
FTY720 bioactivity
To ensure FTY720 remained functional after being loaded and released from the allograft, the in vitro release was repeated to obtain the FTY720 released from coated allografts each day for 5 days; these samples underwent sphingolipid extraction in order to be used in a sphingosine kinase 2 (SPHK2) assay. Briefly, this assay uses SPHK2 and radioactively labeled ATP to phosphorylate FTY720 into FTY720-32P; thus, intact FTY720 that can be phosphorylated as in the body is counted by radioactive activity. To prepare recombinant SPHK2, mouse SPHK2 cDNA was cloned in a pcDNA3.1 vector and expressed in HEK293T cells by transfection. After 2 days, cells were harvested by scraping into a kinase buffer consisting of 20 mM Tris-Cl (pH 7.4), 1 mM 2-mercaptoethanol, 1 mM EDTA, 5 mM sodium orthovanadate, 40 mM β-glycerophosphate, 15 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 10 mM MgCl2, 0.5 mM 4-deoxypyridoxine, 10% glycerol and 0.01 g/L each leupeptin, aprotinin and soybean trypsin inhibitor; the cells were then disrupted with a Dounce homogenizer. The homogenate was clarified by centrifugation at 15,000g, aliquoted and stored at −20°C until use.
SPHK 1 and 2 activity was measured in the kinase buffer. To determine the fractional activity of SPHK1 versus SPHK2, the kinase buffer was supplemented with either 0.5% Triton X-100 or 1 M KCl, respectively. To label FTY720, the kinase buffer was supplemented with FTY720, [γ32P]ATP (10 µM, specific activity=8.3 Ci/mmol), 200 µM KCl and recombinant SPHK2. After 30 min at 37°C, the reaction mixture was extracted with 2 volumes of chloroform/methanol/HCl (100:200:1) and the components in the organic phase were separated by thin layer chromatography using a 1-butanol/acetic acid/water (3:1:1) solvent system. Radiolabeled enzyme products were detected by autoradiography and identified by migration relative to authentic standards. The silica gel containing radiolabeled lipid was scraped into a scintillation vial and quantified with a Beckman Coulter LS 6500 liquid scintillation counter.
Subperiosteal implant and cranial defect surgeries
All animal surgeries were performed according to an approved protocol from the University of Virginia Animal Care and Use Committee. All animals were obtained from Charles River Laboratories International (Wilmington MA, USA). In the subperiosteal implant study, female Sprague Dawley rats (≈270 g) were randomly assigned to three different experimental groups (n=4): uncoated allograft (U), PLAGA coated (C) and 1:200 FTY720 loaded (L). Anesthesia was induced with isoflurane gas and continued with Ketamine/Xylazine (80/8 mg/kg IP). Following anesthetization, the dorsal skin was sterilized with Betadine and 70% ethanol. The longitudinal incision was performed over the dorsum of the skull over the sagittal suture and was made through skin and periosteum. Periosteum was elevated over the parietal and frontal bones to a diameter of 1 cm. The rectangular allograft was placed underneath the periosteum, in direct contact with the parietal bone, centered on the sagittal suture and between the coronal and lamboid sutures. The periosteum was closed with a 5–0 running nylon suture. The skin was closed with a running subcuticular vicryl suture and VetClose™ (Butler Animal Health Supply, Dublin, OH, USA) was applied on the incision. Ketoprofen (3 mg/kg SC) was given after closure and then as needed (once a day) to minimize post-surgery pain. Rats were given free access to food and water and monitored for complications or abnormalities. At 4 weeks post-surgery, rats were anesthetized with 2.5% isoflurane gas and injected in the heart with 1 mL Nembutal.
In the cranial defect study, female Sprague Dawley rats (≈220 g) were randomly assigned to four groups (n=8): uncoated allograft (U), PLAGA coated (C), 1:200 FTY720 loaded (L) and a higher FTY720 loading of 1:40 (H). Animal anesthesia, preparation, post-surgical care and euthanasia at 8 weeks were performed as explained above. A longitudinal incision was made through the skin and periosteum, directly over the sagittal suture. Periosteum was elevated over the parietal and frontal bones to a diameter of 1 cm. A 3-mm round burr was used to create an 8-mm defect in the bone with constant saline irrigation using a Hall® Surgical E900® System and Coolflex® High Speed Drill (CONMED Linvatec, Largo, FL, USA). Thin bone chips still adherent to the dura were carefully elevated with a round knife and removed. The defect was then smoothed with a 1-mm diamond burr. The allograft was placed into the defect and the periosteum was closed over the implant with a 5–0 running nylon suture. The skin was closed with a running subcuticular vicryl suture and VetClose™ was applied over the sutured area.
MicroCT imaging of bone and MICROFIL®
Allografts and animals were imaged using the quantitative microCT. Allografts were scanned with the following parameters: 21 µm voxel size, 55 kVp, 145 µA, medium resolution, 21.5 mm diameter field of view and 200 ms integration time. Animals were scanned with the following parameters: 38 µm voxel size, 55 kVp, 145 µA, medium resolution, 38.9 mm diameter field of view and 200 ms integration time (73 mGy radiation per scan). Rats were scanned biweekly until 4 or 8 weeks for subperiosteal implant or cranial defect studies respectively. 2D images were segmented by drawing the region of interest to comprise only parietal bone by including all bone inside the ridges separating parietal from temporal bone. A slice number was set for each animal throughout the study to include the whole defect and an equal length both anterior and posterior of the defect (≈272 slices). Bone was thresholded at 481.3–2,000 mg hydroxyapatite (HA)/cm3 and bone volume was measured with the microCT software.
Following week 8 in vivo scans for cranial defect rats, all cranial blood vessels were perfused with MICROFIL® MV-122 (Flow Tech, Carver, MA, USA) to image and quantify with microCT (n=3). Rats were euthanized, the heart was exposed and the common carotid arteries were cannulated. The right atrium was incised to allow drainage. Ligating the arteries below the cannulation points allowed direct perfusion of the vasculature in the neck and head region with 2% heparin-saline from both sides simultaneously; each side was perfused with 10 mL in 5 min. Then, 3 mL MICROFIL® mixed 1:1 with dilutent was perfused from each side in 5 min. The solution was allowed to set for 16 h at 4°C. The calvarial sample including the defect and the surrounding parietal bone was harvested, fixated and decalcified as described in the following section. Following decalcification, samples were scanned in air with the following parameters: 21 µm voxel size, 45 kVp, 177 µA, medium resolution, 21.5 mm diameter field of view and 200 ms integration time. Blood vessels in decalcified tissue were thresholded from 164–1,500 mg HA/cm3 and the total blood vessel volume was quantified within a 8 × 9 mm area centered at the defect. The sagittal sinus was excluded from the volume of interest due to its relatively large volume and variation between animals.
3D volume renderings were either done with SCANCO software after segmentation, or OsiriX 3.9 (Pixmeo, Geneva, Switzerland) from DICOM files of the region of interest. OsiriX images were thresholded and colored appropriately with the 16-bit color look-up table (CLUT).
Bone growth qualitative analysis
Bone growth characterization in the cranial defect was organized into 2 types: (1) allograft incorporation and bridging along the interface and (2) host bone healing response in the defect void space. This distinction was made possible by the use of semi-circular allografts in the defect region. Five animals were randomly chosen from each group and their week 2 and 8 MicroCT 3D volumes were scored by at least four blinded evaluators compared to the week zero images. The left side of Table 1 below describes the evaluation of remodeling occurring at the interface on a grading scheme of 1–4, ranging from minimal growth to complete bridging across the entire width of the graft. Table 1’s right side describes bone growth in the defect void region categorized according to amount, aspect ratio (width: height) and location. This growth was determined to directed or undirected towards the graft through the growth aspect ratio. The results of this analysis were used to choose representative microCT 3D images.
Table 1.
Qualitative bone growth diagrammatic representations and scoring descriptions
 |
 |
||
|---|---|---|---|
| Score | host - graft interface growth | Category | bone growth description |
| 1 | Minimal growth at the interface or only graft resorption | A | Longer, undirected growth from or near defect edge (right of line) |
| 2 | Obvious but un-bridged growth at the interface | B | Longer, undirected growth closer to the graft (left of line) |
| 3 | Less bridging growth at interface (left of dashed line) | C | Wider growth towards graft from or near defect edge (right of line) |
| 4 | More bridging growth at interface (both sides of dashed line) | D | Wide growth from edge through the void space (crosses line) |
Histology and immunohistochemisty
Following ex vivo microCT scanning, calvarial samples were fixed in 10% buffered formalin for 7 days and decalcified using an HCl and EDTA decalcifying solution (Richard-Allan Scientific, Kalamazoo, MI, USA) for 3 days at 4°C with agitation. The parietal bone was cut in half, perpendicular to the sagittal suture and centered at the defect or implant. The rostral half was stored in 70% ethanol until paraffin embedding for hematoxylin and eosin (H&E) and Masson’s trichrome staining. The caudal half was flash frozen in Tissue-Tek O.C.T. and cryo-sectioned for α-smooth muscle actin (SMA) staining to visualize mature vessel lumens. Frozen sections were washed (PBS, 0.1% saponin), blocked for 30 min (PBS, 0.1% saponin, 2% bovine serum albumin (BSA)) and immunolabeled for 16 h at 4°C with monoclonal Anti-Actin, α-Smooth Muscle −Cy3™ murine antibody (Sigma Aldrich) diluted 1:500 in (PBS, 0.1% saponin, 0.1% BSA). Labeled sections were then washed and mounted with PBS and glycerol solution (1:1). To quantify changes in vascular remodeling, particularly the recruitment of mural cells, in response to FTY720, obvious lumen formed by SMA+cells was quantified in each tissue section.
Blood cell collection and analysis
Circulating blood monocyte levels in cranial defect animals were quantified immediately, 1 week and 2 weeks after surgery. The rat’s tail vein was incised to achieve a slow, steady bleed. After the first blood was wiped with gauze, 200 µL blood was drawn and mixed with 10 µL EDTA (0.5 M). This solution was sampled and quantified with HEMAVET® 950FS (Drew Scientific Group, Waterbury, CT, USA).
Statistical significance
Results are presented as mean±standard error of the mean. All statistical analysis was performed using a one-way General Linear ANOVA, followed by Tukey’s test for pairwise comparisons with Minitab 16 (Minitab, State College, PA, USA). Significance was asserted at p<0.05 unless otherwise stated.
Results
FTY720 loading, release and bioactivity from polymer coating
To quantify and characterize the nature of the allograft drug delivery system, in vitro release products from PLAGA coated, FTY720 loaded (1:200 drug: PLAGA) grafts were collected and analyzed either with sphingosine kinase 2 assay for bioactivity or with a HPLC-MS for quantity. Figure 1a shows the bioactivity of the released FTY720 after being loaded in the polymer coating. SPHK2 normally phosphorylates the prodrug FTY720 in vivo to FTY720-P, which then acts as a potent agonist for selective S1P receptors. In this assay, only FTY720 able to react with SPHK2 is phosphorylated with radioactive 32P-ATP and measured. Quantified cumulative FTY720 release over 5 days showed a relatively high amount after 1 day and steady additional release each day after (Fig. 1b). After 5 days, the PLAGA coating released over 50% (2.4 µg) of the encapsulated FTY720 (4.5 µg). The release profile closely follows the amount of FTY720 released and labeled in Fig. 1a.
Fig. 1.
FTY720 bioactivity and release from a PLAGA-coated calvarial allograft. a After release, pro-drug FTY720 could be phosphorylated and radiolabeled at detectable levels with a sphingosine kinase 2 assay. b FTY720 released from a graft in vitro quantified with HPLC-MS (1:200 loading). FTY720 quantity released after 1 day has been shown to promote vascular remodeling in vivo
Subperiosteal implant induction of bone growth
Subperiosteal bone implants after 4 weeks are shown in Fig. 2 as ex vivo MicroCT 3D renderings with arrows highlighting new bone formation in the interfacial space between the allograft and host skull. FTY720-loaded grafts induced bridging bone growth all along the surface, while controls only saw the occasional raised bone mass from the skull. Masson’s trichrome staining of the same samples highlights the bone bridging in the interfacial space of FTY720 grafts, while controls had slight growth only at the edges where the distance from graft to host bone was the shortest.
Fig. 2.
Qualitative assessment of subperiosteally implanted allograft integration with host calvaria; 1:200 FTY720-loaded grafts had more interfacial bone growth (arrows) compared to Uncoated or Coated control groups. Representative images at week 4: a MicroCT 3D renderings and b Masson’s trichrome staining
Bone growth and allograft integration in critical size cranial defect
Representative images of calvaria at weeks 0, 2 and 8 post-surgery for the three experimental conditions, as well as a higher loading of FTY720 in the polymer coating (1:40 w:w) are shown in Fig. 3a. Both the uncoated and PLAGA coated controls had some bone growth along the side edges of the defect and minor remodeling of the allograft. Bone regression around the host sagittal suture was common. In contrast, FTY720 groups had almost complete hos–graft bone bridging as well as directed bone growth in the void space. Induced bone growth was often drawn towards the allograft’s sagittal suture dentated edge, which has a higher surface area of bone and therefore more drug-loaded polymer coating than other graft edges.
Fig. 3.
Bone growth and remodeling in a cranial defect area over 8 weeks. a Representative in vivo MicroCT shows FTY720 groups had improved host-graft bridging and directed bone growth in defect space. Controls had undirected bone growth at the defect edges. b Quantification of total bone growth in and around defect at biweekly time points. FTY720 spurs a dose-dependent bone healing response at weeks 2 and 4. By week 6, coated control group’s growth increased while FTY720 groups growth slowed. Bars indicate significance between groups (p<0.05 or *p<0.10)
Total bone volume growth in and directly around, the defect is shown in Fig. 3b for each biweekly time point. At week 2, FTY720 groups had more bone growth compared to controls. The higher dosage of FTY720 (1:40) accelerated bone growth significantly compared to both uncoated and coated controls, while the lower dosage of FTY720 (1:200) was significantly higher than the coated control. By 4 weeks, the lower dosage of FTY720 growth was only slightly higher than the controls and not significantly so. However, 1:40 FTY720 continued to accelerate bone volume growth and was significantly higher than coated control. Between weeks 4 and 6, the lower dosage of FTY720 (1:200) continued growth, while the 1:40 FTY720 group growth slowed down. Despite the fact that both FTY720 treatment groups maintained the higher growth trend, the difference was not significant. Between weeks 6 and 8, all groups saw minimal bone growth changes.
To assess allograft bridging at the host skull interface, as well as host bone growth response in the void space, qualitative bone changes were sorted into categories and evaluated as described in Table 1 of the Methods. Host-allograft interface bone growth and bridging are characterized in Fig. 4a for the four experimental groups. Healing from week 2 to 8 clearly increased interfacial growth. After 8 weeks, both FTY720 groups had host-graft interface bridging in 4/5 animals, compared to 2/5 and 3/5 in Uncoated and Coated controls, respectively. High FTY720 had the most animals with extensive integration (2/5). The bone growth pattern in the defect void space is characterized in Fig. 4b at 2 and 8 weeks. At week 2, all groups except high FTY720 saw the majority (3/5) of animals with random growth at the far edge (category A). High FTY720 also had the highest number of animals with wider, directed growth on the defect void edge (category C). No group had any animals with extensive, directed growth. By week 8, there was a shift in the types of growth from random to directed growth towards the allograft. FTY720 groups promoted extensive directed growth throughout the void from the far defect edge (category D). Controls had mostly random or less directed growth with 1/5 or fewer animals with extensive, directed growth.
Fig. 4.
Qualitative analysis of bone growth and remodeling at the host-graft interface and in the defect void space. a All groups saw increased bridging and growth from weeks 2 to 8. FTY720-loaded groups had the most extensive bridging. b Defect void space growth was characterized through direction and location. All groups except for high FTY720 had mostly random growth along the far edge at week 2. By week 8, FTY720 groups had induced extensive directed growth towards the graft from the far edge of the defect
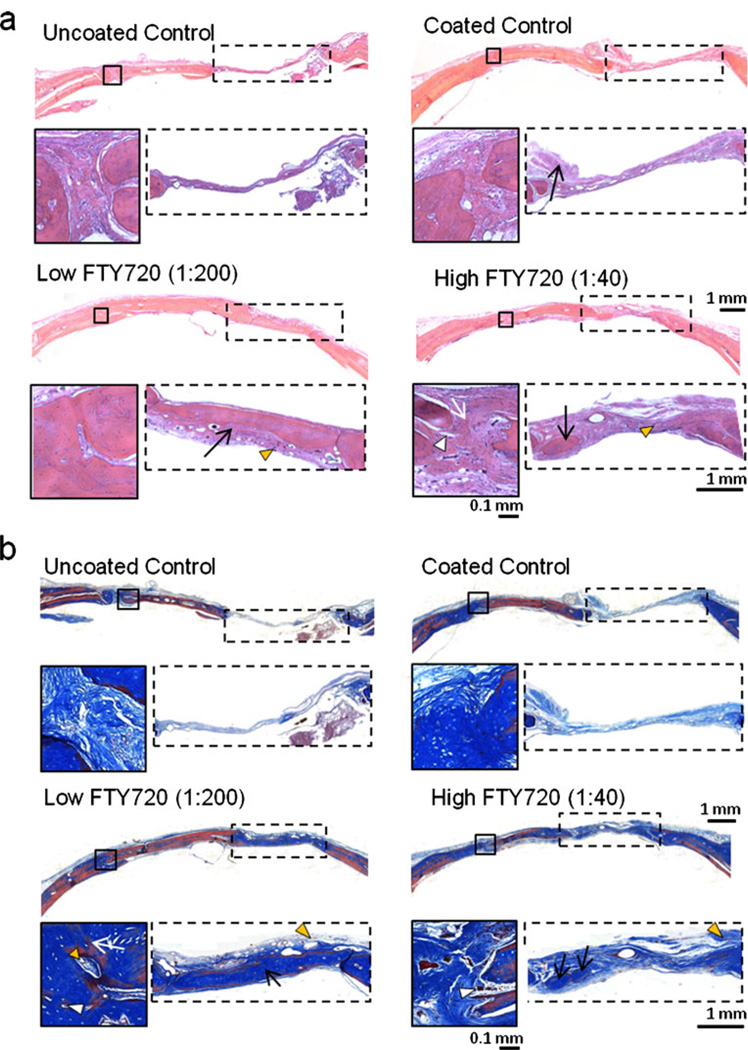
Histological examination and evaluation of the cranial defect growth stained with H&E and Masson’s trichrome after 8 weeks is shown in Fig. 5. From whole tissue sections, the defect void space is still evident in the control samples and the tissue bridging the graft and host bone lacks mature cortical bone. In contrast, the FTY720 groups have mature bone formation at the opposite edge of the defect, in the original void space of the defect and at the host–graft bridge (black box with white arrows showing bridging). The edges of the graft in the control groups are clearly evident and show less incorporation into native bone, compared to graft edges being well integrated in the FTY720 groups. From the Masson’s trichrome stain, graft bone has been encapsulated with collagen matrix (light blue), denoting inclination for further calcification and strengthening. The presence of blood vessels (orange triangles) throughout the periosteum and soft tissue show the region is vascularized, which is a key feature for graft incorporation. Additionally, the presence of osteoclasts and osteoblasts (white triangles) indicate active remodeling in the region.
Fig. 5.
Histological analysis of the cranial defect and allograft area at week 8 with a H&E and b Masson’s trichrome stain. In the FTY720 groups, the allograft shows better incorporation with native bone tissue compared to controls. Host-graft interfacial bridging (black box) was successful in FTY720 groups (white arrow); similar bridging is absent in the control groups. Additionally, in the void space (dotted box), there is increased bone formation in the FTY720 groups compared to the uncoated control (black arrows). Vessel formation near the host-graft interface (orange triangles) and the presence of osteoids and osteoblasts stained dark brown (white triangles) also suggests active remodeling at the bone-graft interface
To explore FTY720’s systemic effect on circulating blood cells and more specifically, osteoclast precursor recruitment, animal blood samples taken immediately after surgery, 1 week and 2 weeks were analyzed for blood monocyte levels and represented in Fig. 6 as percent change from week 0. After 1 week, high FTY720 loading resulted in statistically significant increased monocytes compared to controls but they still remained within the normal range.
Fig. 6.
Monocytes in blood immediately after surgery and in the following weeks. High FTY720 loading leads to elevated circulating monocyte levels at both weeks 1 and 2. * indicates significance to all other groups (p<0.05)
Blood vessel growth evaluation
Blood vessel growth and microvascular maturation are both vital for guiding and maintaining healthy bone growth. Smooth muscle cell investment and support of blood vessels was visualized with α-smooth muscle actin (SMA) fluorescent labeling in cranial defect tissue sections and quantified. Figure 7a shows labeled images of the periosteum region above the graft-void interface. Visible blood vessels with labeled smooth muscle cell support were mainly located in the periosteum and connective void tissue. Vessel lumen shape and diameter varied due to non-perpendicular orientation to the sectioning plane. Therefore, only the number of SMA+ lumens was counted to represent the average total number of mature blood vessels bisecting the middle of the defect, as shown in Fig. 7b. There was no significant difference between any groups at week 8.
Fig. 7.
Fluorescent staining of SMA+blood vessels in the cranial defect area. a Fluorescent images of SMA+blood vessels from the periosteum of the graft-–efect interface are indicative of vessel formation and vascularization of the bone tissue. b Quantification of the total number of SMA+blood vessels resulted in no significant difference between all groups at week 8 post surgery (p=0.73)
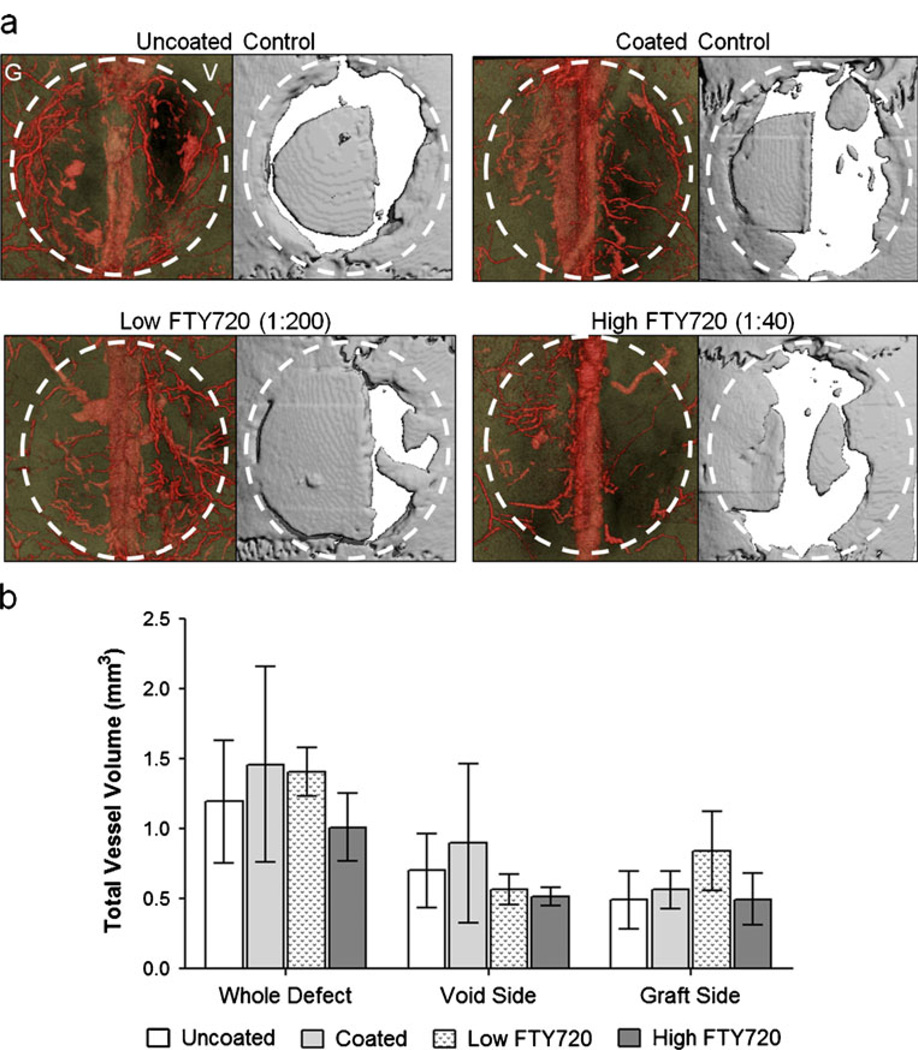
To visualize and quantify the effect of FTY720 on the vascular response, the animal’s head was perfused with radiopaque MICROFIL® and imaged with microCT. Representative 3D renderings of the blood vessel network (diameter>20 µm) and the cranial defect area are shown in Fig. 8a. From comparison of the blood vessel network and bone graft location, it appears that few blood vessels are able to penetrate the actual allograft in all groups. In particular, the control groups had poor graft infiltration, as there are only visible blood vessels below the graft. However, the FTY720 groups appear to have some vessel growth in the plane of the allograft and periosteum. Figure 8b shows quantification of the total blood vessel volume in and around the graft area, excluding the sagittal sinus so that its relatively large and inconsistent volume did not skew the data. Between the groups, there was no statistically significant difference in blood vessel volume in the whole area, on the allograft side, or on the void side. By week 8, FTY720 effects on total blood vessel volume and the total number of mature blood vessels have become indistinguishable from control groups through SMA+ or MICROFIL® analysis. However, FTY720 appears to increase blood vessel investment specifically throughout the allograft and the periosteum above the allograft.
Fig. 8.
Total blood vessel growth in the cranial defect area visualized with MicroCT imaging of a radiopaque agent. a MICROFIL® perfused blood vessel network to visualize with MicroCT. Dotted circle approximates original defect, G is the graft side and V is the void side. b Quantification of blood vessels (thresholding of 0–164 mgHA/cm3) showed by week 8, no significant difference was found among the groups
Discussion
This study investigated the effects of local, sustained release of S1P receptor targeted drug FTY720 from cadaver bone allografts in a critical size cranial defect model. S1P, a known immunomodulator, promotes lymphocyte egress (Schwab et al. 2005; Pappu et al. 2007) and induces an anti-inflammatory phenotype in macrophages (Hughes et al. 2008); it also plays a role in regulating angiogenesis by inducing endothelial cell adherence junction assembly (Lee et al. 1999) and endothelial cell migration (Lee et al. 2001; Paik et al. 2001) through S1P1. Several studies have established that S1P1 also influences stem cell migration (Petrie Aronin et al. 2010a; Ratajczak et al. 2010). Thus, it is hypothesized that the mechanism by which FTY720, an S1P1 agonist, accelerates bone formation is a combination of vascular remodeling, progenitor cell recruitment and immunomodulation.
Delivery of FTY720 was achieved with continuous coating of PLAGA surrounding the bone implant. PLAGA has been extensively used for a variety of applications, such as bioactive molecule delivery (Langer and Vacanti 1993), guided bone regeneration (Oh et al. 2006) and as a mechanically compatible scaffold for human trabecular bone applications (Saito et al. 2010). Due to PLAGA’s easily engineered properties, degradation and biocompatibility can be easily controlled for use in biological sites (Hasirci et al. 2001; Reed and Gilding 1981; Lu et al. 1999) with minimal pH changes (Lu et al. 1999) and it has been shown to be osteoconductive (Ishaug et al. 1997). Though FTY720 encapsulation in a PLAGA scaffold allows for a slow sustained release while maintaining the porous structure of the scaffold (Petrie Aronin et al. 2010a) and without disrupting osteoblast function during bone remodeling (Ishaug et al. 1994), it might not last beyond a few weeks. Due to the high surface area to volume ratio, low thickness and 50% hydrophilic glycolic acid composition, the PLAGA coating is expected to completely degrade and release all FTY720 through bulk degradation within 4–8 weeks (Reed and Gilding 1981; Lu et al. 1999).
The success of an allograft implantation is measured by a combination of both new growth and graft integration. In order to quantify both these responses, semicircular allografts were used in the defects. Figure 3 shows that, although initial bone growth is significant in the FTY720 groups compared to uncoated and coated controls, the total bone volume change is no longer significant at 8 weeks. Known patterns of PLAGA degradation and measurements of FTY720 release could potentially explain this effect. PLAGA degradation is characterized with an initial burst, as noted by 50% of the release occurring by day 5 and then a lag before the rate sharply increases again (Reed and Gilding 1981; Lu et al. 1999). Nevertheless, FTY720 does seem to accelerate this bone growth, as reflected in significantly high bone formation in the first few weeks. A recent study that evaluated the effect of FDA approved alloplastic bone grafts in rat cranial defects found that treatment groups increased the rate of graft incorporation and not total new bone formation (Mah et al. 2004). Though the endpoint total bone volume growth in this study is not significantly increased by FTY720 groups, accelerating the rate of bone regeneration as observed in earlier weeks can have long-term beneficial effects towards successful temporal evolution of bone growth. While the uncoated and coated groups exhibit undirected bone formation, it contributes to the total volume and this contributes to the lack of significant total volume difference at week 8. Additionally, the inclusion of the sutured periosteum could have unexpectedly boosted bone growth in the uncoated and coated controls, due to the high level of osteoblast progenitor cells in the cambium layer (Augustin et al. 2007) and defect protection (Lemperle et al. 1998).
To assess the pattern of new bone formation and the quality of implant integration, evaluation included both the defect healing at the allograft interface and the new bone growth in the void region. Such qualitative evaluations have been utilized in previous studies to provide additional insights into the extent of bone regeneration initiated by different treatments (Johnson et al. 1996; Patel et al. 2008). Since the most significant growth was reflected at the end of 2 weeks, this method was applied both at that time point and at the end of the study. All groups demonstrate higher graft integration at week 8 compared to week 2 and the high loaded treatment group exhibits significantly (D) higher extensive graft directed growth after 8 weeks. It appears as though (C) initial acceleration of growth in the void as seen in the week 2 data translates into this extensive growth. As microvascular expansion is crucial for bone regeneration (Collin-Osdoby 1994; Kleinheinz et al. 2005; Murphy et al. 2004), the earlier occurrence of a more developed vasculature in the high loaded groups could potentially be the reason behind more extensive void region growth. Moreover, the assessment of bone formation in the void region indicates that the FTY720-loaded groups have a higher volume of directed growth towards the non-curved edge of the allograft compared to the controls at both time points. The allografts are cut along the sagittal suture and have a dented edge that increases the surface area, causing a higher amount of drug-loaded polymer to accumulate in that region. It is hypothesized that this accumulation causes directed growth resulting in possible bridging. Histological examination of FTY720-loaded groups confirmed the qualitative analysis, as week 8 images showed mature bone growth incorporation with the graft and host bone; defect void space was well filled with mature bone formations and vascularized connective tissue.
Although FTY720 has proven efficacious in enhancing vascular growth and maturation in various animal models, its effects were mostly diminished by week 8 as there was no significant difference in the quantified blood vessel volume and vessel thickness as discerned by SMA staining (Figs. 7 and 8). Previous work where FTY720 promoted arteriogenesis in the dorsal skinfold window chamber was not able to evaluate vascular remodeling beyond 3 weeks of drug application, due to model limitations (Sefcik et al. 2011). Intramembranous bones are more challenging to vascularize than endochondral bones (Sullivan and Szwajkun 1991), so FTY720’s vascular effects may be more important in this study compared to an earlier study in tibial defects (Petrie Aronin et al. 2010b). Patel et al. released VEGF and BMP-2 individually and in combination in the cranial defect without significant effect on blood vessel volume at week 4 (Patel et al. 2008). They concluded that VEGF’s influence may have been more notable at an earlier time point, or it may have been too weak to sustain an enhanced, mature vasculature after 4 weeks. Indeed, it seems that week 8 was also too late to capture differences due to FTY720 treatment in this study. However, the imaging method only allowed for visualization of vessels with a diameter larger than 20 µm, precluding capillary and smaller blood vessel visibility. Therefore, FTY720 effects on microvasculature may have been missed due to imaging limitations. Additionally, the 3D vasculature images clearly show flat shapes that do not resemble vessels, due to MICROFIL® leakage from overly high perfusion pressure; these shapes were not included in the region of interest but this may have introduced error into the blood vessel volume quantification (Bouxsein et al. 2010).
FTY720 could also be aiding in recruiting circulating progenitor cells. In previous work, it was shown that S1P mediates dura stem cell migration in an S1P3 dependent manner, as treatment with S1P increased their migration while treatment with VPC01091, a S1P3 antagonist, did not (Petrie Aronin et al. 2010a). High FTY720 loading in this study increased osteoclast precursor cell circulation (monocyte) levels significantly compared to other groups, although they still remained within the normal range (Fig. 6). Perhaps through a related mechanism, S1P1 agonism has been shown to affect osteoclastic activity in a postmenopausal osteoporotic mouse model and the use of FTY720 resulted in decreasing the attachment of osteoclasts to the bone surface (Ishii et al. 2009). It has also been established that FTY720 helps intestinal allograft integration by preventing T cell infiltration (Kimura et al. 2003). Both vascularization and progenitor cell recruitment are known to increase the rate of bone regeneration via allograft integration and remodeling and FTY720 is known to have an influence on both these processes. The increased acceleration of bone regeneration observed at the end of week 2 could be attributed to a combination of these events.
Conclusion
We have shown that FTY720 can be released in a sustained manner from a PLAGA coating in both subperiosteal and cranial defect implants. Local drug delivery from coated allografts increased bone regeneration rate, graft integration and directed bone growth in the void after 8 weeks of implantation in a critical size cranial defect. FTY720-loaded allografts had dose-dependent effects, suggesting that drug encapsulation and release kinetics can be improved to obtain optimal bone growth profiles. Mechanisms for further exploration include early vascularization promotion, immune cell modulation and osteoclast precursor cell circulation.
Acknowledgements
Thanks to Yugesh Kharel, Kevin Lynch, Tyler Pegoraro, Ji Song, Jose Tomsig and UVA Research and Histology Core for technical expertise and aid. Sources of support for this study include the UVA Coulter Translational research Partnership Program and National institutes of Health grants K01AR052352-05, R01AR056445-02, R01DE019935-02 to Dr. Botchwey. Cynthia Huang is supported by the National Institutes of Health Cardiovascular Training Grant HL007284-34.
Contributor Information
Cynthia Huang, Biomedical Engineering, University of Virginia, PO Box 800759, Health System, Charlottesville, VA 22908, USA.
Anusuya Das, Biomedical Engineering, University of Virginia, PO Box 800759, Health System, Charlottesville, VA 22908, USA.
Daniel Barker, Otolaryngology, University of Virginia, Virginia, USA.
Sunil Tholpady, Plastic and Maxillofacial Surgery, University of Virginia, Virginia, USA.
Tiffany Wang, Biomedical Engineering, University of Virginia, PO Box 800759, Health System, Charlottesville, VA 22908, USA.
Quanjun Cui, Orthopaedic Surgery, University of Virginia, Virginia, USA.
Roy Ogle, LifeNet Health Institute of Regenerative Medicine, Virginia, USA.
Edward Botchwey, Email: botchwey@virginia.edu, Biomedical Engineering, University of Virginia, PO Box 800759, Health System, Charlottesville, VA 22908, USA; Orthopaedic Surgery, University of Virginia, Virginia, USA.
References
- Augustin G, Antabak A, Davila S. The periosteum Part 1: Anatomy, histology and molecular biology. Injury-Int J Care Inj. 2007;38:1115–1130. doi: 10.1016/j.injury.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Boyce T, Edwards J, Scarborough N. Allograft bone. The influence of processing on safety and performance. Orthop Clin North Am. 1999;30:571–581. doi: 10.1016/s0030-5898(05)70110-3. [DOI] [PubMed] [Google Scholar]
- Chen NT, Glowacki J, Bucky LP, Hong HZ, Kim WK, Yaremchuk MJ. The roles of revascularization and resorption on endurance of craniofacial onlay bone grafts in the rabbit. Plast Reconstr Surg. 1994;93:714–724. [PubMed] [Google Scholar]
- Collin-Osdoby P. Role of vascular endothelial cells in bone biology. J Cell Biochem. 1994;55:304–309. doi: 10.1002/jcb.240550306. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: What they can offer and what they cannot. J Bone Joint Surg Br. 2007;89:574–579. doi: 10.1302/0301-620X.89B5.19039. [DOI] [PubMed] [Google Scholar]
- Delloye C, de Nayer P, Allington N, Munting E, Coutelier L, Vincent A. Massive bone allografts in large skeletal defects after tumor surgery: a clinical and microradiographic evaluation. Arch Orthop Trauma Surg. 1988;107:31–41. doi: 10.1007/BF00463522. [DOI] [PubMed] [Google Scholar]
- DePaula CA, Truncale KG, Gertzman AA, Sunwoo MH, Dunn MG. Effects of hydrogen peroxide cleaning procedures on bone graft osteoinductivity and mechanical properties. Cell Tissue Bank. 2005;6:287–298. doi: 10.1007/s10561-005-3148-2. [DOI] [PubMed] [Google Scholar]
- Enneking WF, Campanacci DA. Retrieved human allografts: a clinicopathological study. J Bone Joint Surg Am. 2001;83-A:971–986. [PubMed] [Google Scholar]
- Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36:S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Hasirci V, Lewandrowski K, Gresser JD, Wise DL, Trantolo DJ. Versatility of biodegradable biopolymers: degradability and an in vivo application. J Biotechnol. 2001;86:135–150. doi: 10.1016/s0168-1656(00)00409-0. [DOI] [PubMed] [Google Scholar]
- Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36:17–28. doi: 10.1002/(sici)1097-4636(199707)36:1<17::aid-jbm3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Ishaug SL, Yaszemski MJ, Bizios R, Mikos AG. Osteoblast function on synthetic biodegradable polymers. J Biomed Mater Res. 1994;28:1445–1453. doi: 10.1002/jbm.820281210. [DOI] [PubMed] [Google Scholar]
- Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EE, Urist MR. Human bone morphogenetic protein allografting for reconstruction of femoral nonunion. Clin Orthop Relat Res. 2000;371:61–74. doi: 10.1097/00003086-200002000-00008. [DOI] [PubMed] [Google Scholar]
- Johnson KD, Frierson KE, Keller TS, Cook C, Scheinberg R, Zerwekh J, Meyers L, Sciadini MF. Porous ceramics as bone graft substitutes in long bone defects: A biomechanical, histological, and radiographic analysis. J Orthop Res. 1996;14:351–369. doi: 10.1002/jor.1100140304. [DOI] [PubMed] [Google Scholar]
- Kimura T, Hasegawa T, Nakai H, Azuma T, Usui N, Sasaki T, Okada A. FTY720 reduces T-cell recruitment into murine intestinal allograft and prevents activation of graft-infiltrating cells. Transplantation. 2003;75:1469–1474. doi: 10.1097/01.TP.0000058816.13525.92. [DOI] [PubMed] [Google Scholar]
- Kleinheinz J, Stratmann U, Joos U, Wiesmann HP. VEGF-activated angiogenesis during bone regeneration. J Oral Maxillofac Surg. 2005;63:1310–1316. doi: 10.1016/j.joms.2005.05.303. [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, Wu M, Morales-Ruiz M, Sessa WC, Alessi DR, Hla T. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell. 2001;8:693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- Lemperle SM, Calhoun CJ, Curran RW, Holmes RE. Bony healing of large cranial and mandibular defects protected from soft-tissue interposition: A comparative study of spontaneous bone regeneration, osteoconduction, and cancellous autografting in dogs. Plast Reconstr Surg. 1998;101:660–672. doi: 10.1097/00006534-199803000-00013. [DOI] [PubMed] [Google Scholar]
- Lu L, Garcia CA, Mikos AG. In vitro degradation of thin poly (DL-lactic-co-glycolic acid) films. J Biomed Mater Res. 1999;46:236–244. doi: 10.1002/(sici)1097-4636(199908)46:2<236::aid-jbm13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Mah J, Hung J, Wang J, Salih E. The efficacy of various alloplastic bone grafts on the healing of rat calvarial defects. Eur J Orthod. 2004;26:475–482. doi: 10.1093/ejo/26.5.475. [DOI] [PubMed] [Google Scholar]
- Mankin HJ, Hornicek FJ, Raskin KA. Infection in massive bone allografts. Clin Orthop Relat Res. 2005;432:210–216. doi: 10.1097/01.blo.0000150371.77314.52. [DOI] [PubMed] [Google Scholar]
- Mokbel N, Bou Serhal C, Matni G, Naaman N. Healing patterns of critical size bony defects in rat following bone graft. Oral Maxillofac Surg. 2008;12:73–78. doi: 10.1007/s10006-008-0107-7. [DOI] [PubMed] [Google Scholar]
- Murphy WL, Simmons CA, Kaigler D, Mooney DJ. Bone regeneration via a mineral substrate and induced angiogenesis. J Dent Res. 2004;83:204–210. doi: 10.1177/154405910408300304. [DOI] [PubMed] [Google Scholar]
- Oh SH, Kim JH, Kim JM, Lee JH. Asymmetrically porous PLGA/Pluronic F127 membrane for effective guided bone regeneration. J Biomater Sci Polym Ed. 2006;17:1375–1387. doi: 10.1163/156856206778937253. [DOI] [PubMed] [Google Scholar]
- Paik J, During A, Harrison EH, Mendelsohn CL, Lai K, Blaner WS. Expression and characterization of a murine enzyme able to cleave beta-carotene. The formation of retinoids. J Biol Chem. 2001;276:32160–32168. doi: 10.1074/jbc.M010086200. [DOI] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebay A, Bonder CS, Pitson SM. Stem cell regulation by lysophospholipids. Prostaglandins Other Lipid Mediat. 2007;84:83–97. doi: 10.1016/j.prostaglandins.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Petrie Aronin CE, Sefcik LS, Tholpady SS, Tholpady A, Sadik KW, Macdonald TL, Peirce SM, Wamhoff BR, Lynch KR, Ogle RC, Botchwey EA. FTY720 promotes local microvascular network formation and regeneration of cranial bone defects. Tissue Eng Part A. 2010a;16:1801–1809. doi: 10.1089/ten.tea.2009.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie Aronin CE, Shin SJ, Naden KB, Rios PD, Jr, Sefcik LS, Zawodny SR, Bagayoko ND, Cui Q, Khan Y, Botchwey EA. The enhancement of bone allograft incorporation by the local delivery of the sphingosine 1-phosphate receptor targeted drug FTY720. Biomaterials. 2010b;31:6417–6424. doi: 10.1016/j.biomaterials.2010.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinholt EM, Solheim E, Talsnes O, Larsen TB, Bang G, Kirkeby OJ. Revascularization of calvarial, mandibular, tibial, and iliac bone grafts in rats. Ann Plast Surg. 1994;33:193–197. doi: 10.1097/00000637-199408000-00012. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A, Ratajczak J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AM, Gilding DK. Biodegradable polymers for use in surgery — poly(glycolic)/poly(Iactic acid) homo and copolymers: 2. In vitro degradation Polymer. 1981;22:494–498. [Google Scholar]
- Ryu J, Kim HJ, Chang EJ, Huang H, Banno Y, Kim HH. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006;25:5840–5851. doi: 10.1038/sj.emboj.7601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito E, Kang H, Taboas JM, Diggs A, Flanagan CL, Hollister SJ. Experimental and computational characterization of designed and fabricated 50:50 PLGA porous scaffolds for human trabecular bone applications. J Mater Sci Mater Med. 2010;21:2371–2383. doi: 10.1007/s10856-010-4091-8. [DOI] [PubMed] [Google Scholar]
- Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:299–308. [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Sefcik LS, Aronin CE, Awojoodu AO, Shin SJ, Mac Gabhann F, MacDonald TL, Wamhoff BR, Lynch KR, Peirce SM, Botchwey EA. Selective activation of sphingosine 1-phosphate receptors 1 and 3 promotes local microvascular network growth. Tissue Eng Part A. 2011;17:617–629. doi: 10.1089/ten.tea.2010.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers RS, Zhang R, Glasson SS, Kim HD, Peluso D, D'Augusta DA, Beckwith K, Morris EA. Repair of articular cartilage defects one year after treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2) J Bone Joint Surg Am. 2000;82:151–160. doi: 10.2106/00004623-200002000-00001. [DOI] [PubMed] [Google Scholar]
- Sharma S, Mathur AG, Pradhan S, Singh DB, Gupta S. Fingolimod (FTY720): First approves oral therapy for multiple sclerosis. J Pharmacol Pharmacother. 2011;2:49–51. doi: 10.4103/0976-500X.77118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson S, Emery SE, Goldberg VM. Factors affecting bone graft incorporation. Clin Orthop Relat Res. 1996;324:66–74. doi: 10.1097/00003086-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Sullivan WG, Szwajkun PR. Revascularization of cranial versus iliac crest bone grafts in the rat. Plast Reconstr Surg. 1991;87:1105–1109. doi: 10.1097/00006534-199106000-00013. [DOI] [PubMed] [Google Scholar]
- Thompson RC, Jr, Pickvance EA, Garry D. Fractures in large-segment allografts. J Bone Joint Surg Am. 1993;75:1663–1673. doi: 10.2106/00004623-199311000-00011. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005;435:36–42. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- Yazici C, Takahata M, Reynolds DG, Xie C, Samulski RJ, Samulski J, Beecham EJ, Gertzman AA, Spilker M, Zhang X, O'Keefe RJ, Awad HA, Schwarz EM. Self-complementary AAV2 5-BMP2-coated Femoral Allografts Mediated Superior Bone Healing Versus Live Autografts in Mice With Equivalent Biomechanics to Unfractured Femur. Mol Ther. 2011 doi: 10.1038/mt.2010.294. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114:155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]