Abstract
Progression of hyperglycemia-induced renal injury is a contributing factor for diabetic nephropathy (DN)-induced end-stage renal disease (ESRD), and development of novel therapeutic strategies that act early to prevent progression of DN and ESRD are important. We examined the efficacy and mechanism(s) of suramin on hyperglycemia-induced renal injury before development of overt histological damage. Two groups of male Sprague-Dawley rats received streptozotocin (STZ) and one group received saline. Three weeks later, one STZ group received suramin (10 mg/kg). All animals were euthanized 1 week later (4 weeks). Although there was a decrease in creatinine clearance between control and STZ ± suramin rats, there was no difference in creatinine clearance between STZ rats ± suramin intervention. Liquid chromatography-tandem mass spectroscopy-based analysis revealed increases in urinary proteins that are early indicators of DN (e.g., cystatin C, clusterin, cathepsin B, retinol binding protein 4, and peroxiredoxin-1) in the STZ group, which were blocked by suramin. Endothelial intracellular adhesion molecule-1 (ICAM-1) activation, leukocyte infiltration, and inflammation; transforming growth factor-β1 (TGF-β1) signaling; TGF-β1/SMAD-3-activated fibrogenic markers fibronectin-1, α-smooth muscle actin, and collagen 1A2; activation of proinflammatory and profibrotic transcription factors nuclear factor-κB (NF-κB) and signal transducer and activator of transcription factor-3 (STAT-3), respectively, were all increased in STZ rats and suramin blocked these changes. In conclusion, delayed administration of suramin attenuated 1) urinary markers of DN, 2) inflammation by blocking NF-κB activation and ICAM-1-mediated leukocyte infiltration, and 3) fibrosis by blocking STAT-3 and TGF-β1/SMAD-3 signaling. These results support the potential use of suramin in DN.
Introduction
The prevalence of diabetes mellitus has been increasing worldwide and is estimated to continue in the future (Mokdad et al., 2003). Hyperglycemia-induced diabetic nephropathy (DN) is a serious complication and is the most common cause of end-stage renal disease (ESRD), which has more than doubled in the past decade and at present accounts for approximately 50% of all ESRD, increasing renal replacement therapy (Molitch et al., 2004). This is partly due to insufficient knowledge of the pathophysiology initiated by hyperglycemia, the resulting DN, and its detection at a late stage. Some of the most important events in the pathogenesis of hyperglycemia-induced renal injury and DN are oxidative stress, inflammation, and fibrosis (Kanwar et al., 2011; Wang et al., 2011). Current therapies directed to delaying the progression of DN include 1) intensive glycemic and optimal blood pressure control, 2) interruption of the renin-angiotensin system through the use of angiotensin-converting enzyme inhibitors and angiotensin type 1 receptor blockers, and 3) dietary modification, flavonoid administration, and cholesterol-lowering agents (Sharma et al., 2008; Balakumar et al., 2009). However, the renal protection provided by these therapeutic modalities is insufficient to control the progression of DN, partly because of their intervention at a late stage. Therefore, it is prudent to obtain further understanding of the signaling pathways involved in the early stage of hyperglycemia-induced renal pathogenesis and develop pharmacological agents that target these key signaling pathways in DN and ameliorate the progression of this disease.
In this regard, delayed administration of suramin, a drug that has been used extensively in humans to treat trypanosomiasis, was recently shown to decrease renal injury in various animal models of acute kidney injury and renal inflammation and fibrosis in chronic kidney disease (Zhuang et al., 2009; Liu et al., 2011a; Korrapati et al., 2012). Because patients with DN usually have some degree of renal inflammation and fibrosis (Mathew et al., 2011; Najafian et al., 2011), it would be clinically useful if delayed administration of suramin prevents inflammation and fibrosis. Hence we tested this hypothesis in a rat model of STZ-induced diabetes and examined possible mechanisms of action. It is important to note that the STZ model used in this study represents the early effects of hyperglycemia on the kidney before overt renal histological changes and DN. In addition to testing the above-mentioned hypothesis, another important goal of this study was to perform urinalysis by LC-MS/MS to identify indicators of early-stage DN and potential novel proteins in this model and to determine whether there is any effect of suramin on them.
Materials and Methods
Animals and Treatment.
Male Sprague-Dawley rats (Harlan Laboratories, Madison, WI), 8 weeks of age (180–200 g), were housed in temperature-controlled conditions under a light/dark photocycle with food and water supplied ad libitum. Food was removed from rats for 16 h, and rats were divided randomly into five different groups. The first group (n = 5) was injected with sterile water intravenously, 3 weeks after saline treatment; the second group (n = 5) received 10 mg/kg suramin (intravenously, dissolved in sterile water) 3 weeks after the initial saline treatment; and the third, fourth, and fifth groups of rats (n = 5) were injected with 55 mg/kg i.v. STZ in saline (Covington and Schnellmann, 2012). After STZ injection, rats were given drinking water supplemented with sucrose (15 g/l) for 48 h to limit mortality as stores of insulin are released from damaged pancreatic islet. After 24 h, diabetes was confirmed in STZ-treated rats by tail vein plasma glucose levels. The third group of rats received a single dose of sterile water intravenously, 3 weeks after initial STZ treatment, the fourth group of rats received 10 mg/kg suramin (intravenously, dissolved in sterile water) 3 weeks after the initial STZ treatment, and the fifth group of rats received 5 mg/kg suramin (intravenously, dissolved in sterile water) 3 weeks after initial STZ treatment. One week later rats were placed in metabolism cages for 24 h and then euthanized. Serum and urine were collected, and kidneys were harvested. One kidney was fixed in formaldehyde and stained with hematoxylin and eosin for morphological assessment. Unstained slides were used for immunohistochemical analysis. The other kidney was frozen at −80°C until further analysis. All animal and treatment protocols were in compliance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (Institute of Laboratory Animal Resources, 1996) and were approved by our institutional animal care and use committee.
Urine volumes (24 h) were recorded, renal function was monitored by measuring serum and urine creatinine using a creatinine assay kit (BioAssay Systems, Hayward, CA) as per the manufacturer's instructions, and creatinine clearance was calculated. Renal tissues were fixed in 4.5% buffered formalin, dehydrated, and embedded in paraffin. For general histology, sections were stained with hematoxylin/eosin. For immunohistochemical analysis of COL1A2, the manufacturer's protocol was followed (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Chemicals.
Unless stated otherwise, all chemicals and biochemicals were purchased from Sigma-Aldrich (St. Louis, MO). All LC-MS/MS reagents were LC-grade pure and purchased from Waters (Milford, MA). Sources of antibodies were as follows: 1) goat polyclonal anti-kidney injury molecule-1 (KIM-1), R&D Systems (Minneapolis, MN); 2) rabbit polyclonal anti-neutrophil gelatinase-associated lipocalin-2 (NGAL) and mouse monoclonal anti-transforming growth factor-β1 (TGF-β1), Abcam Inc. (Cambridge, MA); 3) rabbit polyclonal anti-caspase-3, Enzo Life Sciences (Farmingdale, NY); 4) rabbit polyclonal anti-intracellular adhesion molecule-1 (ICAM-1), mouse monoclonal fibronectin-1, and goat polyclonal anti-collagen 1A2, Santa Cruz Biotechnology (Santa Cruz, CA); 5) mouse monoclonal anti-α-smooth muscle actin, Sigma-Aldrich; 6) rabbit monoclonal anti-SMAD-3, anti-phospho-SMAD-3, anti-extracellular regulated kinase or mitogen-activated protein kinase 44/42 (ERK1/2), anti-phospho-ERK1/2, anti-signal transducer and activator of transcription factor-3 (STAT-3), anti-phospho-STAT-3 and anti-phospho-p65, Cell Signaling Technology (Danvers, MA); and 7) loading control glyceraldehyde 3-phosphate dehydrogenase (GAPDH), Fitzgerald International Inc. (Acton, MA). Anti-goat secondary antibody conjugated with horseradish peroxidase was purchased from Millipore Corporation (Billerica, MA), and anti-rabbit and anti-mouse secondary antibodies conjugated with horseradish peroxidase were obtained from Thermo Fisher Scientific (Waltham, MA).
Urinary Biomarker Identification Using LC-MS/MS.
Frozen urine aliquots were thawed at 37°C for 10 min and centrifuged for 10 min at 1000g and 4°C, and total urinary protein and creatinine values were measured. The sample volume used for trypsin digestion and subsequent proteomic analysis was calculated by normalizing total urinary protein to both urine volume and urine creatinine to eliminate biological variability. Urine samples were diluted with an equal volume of 0.2% (w/v) Rapigest (Waters) in 100 mM ammonium bicarbonate, and 200 ng of recombinant HIV gp160 was added to each sample as an internal standard for downstream analysis. Samples were reduced in the presence of dithiothreitol (10 mM final concentration) by incubation for 60 min at 37°C. Next, cysteine residues were alkylated by incubation with 15 mM iodoacetamide in the dark at room temperature for 30 min. Trypsin was then added at a ratio of 1:20 to total protein, and the samples were digested overnight at 37°C. To stop the digestion, samples were acidified by the addition of 0.1% (v/v) formic acid to a final volume of 1 ml. Each sample was then chromatographically separated using offline reverse-phase solid-phase extraction (SPE) with Strata-X SPE columns (Phenomenex, Torrance, CA). In brief, the SPE column was activated with methanol and equilibrated with 0.1% formic acid. The sample was loaded on the column and washed with a solution of 5% acetonitrile in 0.1% formic acid. Serial elutions were performed with eluents of 30, 40, and 60% acetonitrile in 0.1% formic acid. Sample eluates were dried down in a centrifugal vacuum concentrator and reconstituted with mobile phase A (98% water, 0.2% formic acid, and 2% acetonitrile) used for the next phase of chromatography. Peptide concentrations were estimated in each sample fraction using the absorbance at 280 nm (NanoDrop), and the volumes of each fraction were normalized across samples such that the most concentrated sample had a calculated concentration of 0.2 μg/μl. Samples were separated with an online 2D+ NanoLC system (Eksigent, Dublin, CA). In brief, 5 μl of each reconstituted sample fraction was loaded onto a 100 μm × 1 cm C18 (100 Å with 5-mm particles) trap column (Acclaim PepMap 100; Thermo Fisher Scientific), and separated on a 75 μm × 15 cm C18 (300 Å with 3-μm particles) analytical column (Acclaim PepMap 100; Thermo Fisher Scientific). Reverse-phase separation was accomplished using a 35-min gradient from 5% acetonitrile, 0.2% formic acid to 85% acetonitrile, 0.2% formic acid. Data acquisition was performed with a TripleTOF 5600 System (Applied Biosystems/MDS Sciex, Foster City, CA) fitted with a nanospray source. For instrument-dependent acquisition, precursor ion scans were acquired for 250 ms with up to 20 product ion scans of 50 ms if precursors were 300 to 1250 m/z, exceeded 125 cps, and had a 2+ to 5+ charge state. Dynamic exclusion was set to 5 s (corresponding to one-half peak width), and isotopes within 4 Da and with a mass tolerance of 50 mDa were excluded. Acquired raw data were converted to the MGF file format using the Applied Biosystems/MDS Sciex converter (version 1.1). Resulting peak lists were searched using the Mascot search engine against the UniProt Swiss-Prot database (2011_6 release) for the taxonomy Rattus norvegicus including the Global Proteome Machine Organization common Repository of Adventitious Proteins (cRAP) database. The enzyme specified was trypsin, and up to two missed cleavages were allowed. The only fixed modification was carbamidomethyl (C). Variable modifications included acetylation (N-term), deamidation (NQ), Gln → pyro-Glu (N-term Q), and oxidation (M). Parent ion tolerance was 20 ppm and the fragment ion tolerance was 0.5 Da. Scaffold (version 3.2.0; Proteome Software Inc., Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Protein identifications were accepted if they could be established at greater than 50% peptide probability and greater than 99% protein probability and contained at least two identified peptides as specified by the Peptide Prophet algorithm (Keller et al., 2002; Nesvizhskii et al., 2003). On the basis of these criteria, a false discovery rate of 2.2% was achieved (false discovery rate ≤5% is usually desirable), which means that the protein identification is highly reliable and accurate (Keller et al., 2002; Nesvizhskii et al., 2003). Unweighted spectral counts for each sample were exported. The unweighted spectral counts for each protein were divided by the unweighted spectral counts for the HIV gp160 protein from each sample.
Assessment of Renal Inflammation.
Renal inflammation was assessed by measuring leukocyte (neutrophils and monocytes) infiltration using the naphthol AS-D chloroacetate esterase kit (Sigma-Aldrich), and immunohistochemical analysis was performed as per the manufacturer's protocol. To quantify leukocyte infiltration, a total of 25 fields (original magnification, 20×) in the outer stripe of outer medulla (OSOM) were examined, and results are expressed as the total number of leukocytes in all the fields.
Immunoblot Analysis.
Rat kidney cortex tissue was homogenized in 5 volumes of protein lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl, pH 7.4; 1 mM EDTA; 1 mM EGTA; 2 mM sodium orthovanadate; 0.2 mM phenylmethylsulfonyl fluoride; 1 mM HEPES, pH 7.6; 1 μg/ml leupeptin; and 1 μg/ml aprotinin) using a Polytron homogenizer. The homogenate was stored on ice for 10 min and then centrifuged at 7500g for 5 min at 4°C. The supernatant was collected, and protein was determined using a bicinchoninic acid kit (Sigma-Aldrich) with bovine serum albumin as the standard. Proteins (50–75 μg) were separated on 4 to 20% gradient SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked either in 5% dried milk or BSA in TBST (0.1% Tween 20 in 1× Tris-buffered saline) and incubated with 1:1000 dilutions of anti-KIM-1, anti-NGAL, anti-caspase-3, anti-TGF-β1, anti-SMAD-3, anti-phospho-SMAD-3, anti-α-SMA, anti-ERK1/2, anti-phospho-ERK1/2, anti-STAT-3, anti-phospho-STAT-3, anti-phospho-p65, and anti-GAPDH and 1:100 dilutions of anti-ICAM-1 and anti-fibronectin-1 overnight at 4°C. After incubation for 2 h at room temperature with secondary antibodies (1:2000) conjugated with horseradish peroxidase, membrane proteins were detected by chemiluminescence.
Data and Statistical Analysis.
Data are expressed as means ± S.E.M. (n = 4–5) for all experiments. Multiple comparisons of normally distributed data were analyzed by one-way analysis of variance, as appropriate, and group means were compared using the Student-Newman-Keuls post hoc test. Single comparisons were analyzed by Student's t test where appropriate. The criterion for statistical differences was p ≤ 0.05 for all comparisons.
Results
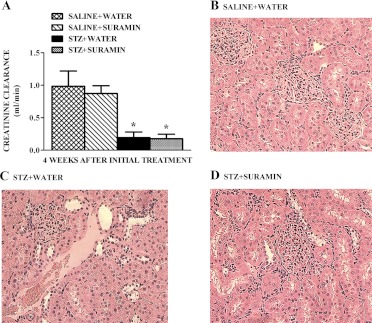
At week 1 and week 4 after either STZ or saline injections, blood glucose levels were greater than 600 mg/dl in the diabetic rats and 133 mg/dl in controls, respectively. Body weights of STZ rats were lower than those of control rats at 3 weeks (206 ± 7 and 305 ± 5 g, respectively). Urinary volume increased greater than 3-fold in STZ rats, and creatinine clearance in STZ rats decreased 80% compared with controls (Fig. 1A). There were no differences in total urinary protein normalized to urine volume and urine creatinine in control and STZ rats (data not shown). Neither 5 nor 10 mg/kg suramin had any effect on body weights, blood glucose, urinary volume, or creatinine clearance in STZ rats. Because suramin (10 mg/kg) alone had no effect on any of these parameters and this dose has been used previously (Liu et al., 2012), we used this dose in all remaining studies.
Fig. 1.
Effect of delayed administration of suramin on renal injury in STZ-induced diabetic rats. The first group was injected with sterile water intravenously, 3 weeks after saline treatment; the second group received 10 mg/kg suramin (intravenously, dissolved in sterile water) 3 weeks after the initial saline treatment; the third group of rats received a single dose of sterile water intravenously, 3 weeks after the initial STZ treatment in saline, intravenously; and the fourth group of rats received 10 mg/kg suramin (intravenously, dissolved in sterile water) 3 weeks after the initial STZ treatment. Rats in all groups were terminated 1 week later after second treatment. Creatinine clearance (A) in nondiabetic and diabetic rats ± suramin intervention, at week 4 after initial treatments was measured. Data are expressed as means ± S.E. (n = 5). *, significantly different from the respective saline controls. (p ≤ 0.05). Representative photomicrographs of hematoxylin and eosin-stained kidney sections from nondiabetic rats (B) and diabetic rats treated with diluent (C) and with suramin (D). All fields were chosen from the OSOM. Original magnification, 200×.
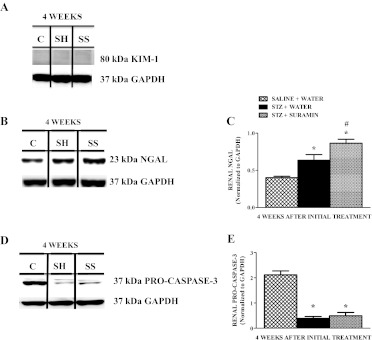
There were no gross histological changes in glomeruli or tubules among control and STZ rat kidneys ± suramin treatment (Fig. 1, B–D). In addition, kidney injury molecule-1 protein expression was not detected in the STZ rat kidneys ± suramin intervention (Fig. 2A). However, renal NGAL levels were increased in STZ rats compared with controls, and suramin-treated STZ rats had slightly higher renal NGAL levels compared with STZ rats (Fig. 2, B and C). We have previously shown increased cleavage of procaspase-3 and apoptosis in rat kidneys 10 weeks after STZ (Covington and Schnellmann, 2012). Likewise, we found equivalent decreases in procaspase-3 in the kidneys of rats after STZ ± suramin treatment 4 weeks after STZ treatment (Fig. 2, D and E). Thus, in this 4-week STZ model, gross renal histology and serum creatinine and urinary protein levels were not changed, renal apoptosis was ongoing, urinary volume increased, and suramin treatment had no effect on apoptosis.
Fig. 2.
Representative Western blot showing KIM-1 (A), NGAL (B), and procaspase-3 (D) protein expressions in kidneys of nondiabetic and diabetic rats ± suramin intervention on week 4 after initial treatments. Densitometric analysis of renal NGAL (C) and procaspase-3 (E) protein expressions in kidneys of nondiabetic and diabetic rats ± suramin intervention at week 4 after initial treatments. Data were normalized by GAPDH, which served as the internal control. Data are expressed as means ± S.E. (n = 4). *, significant from the respective nondiabetic saline + water controls. #, significant from the respective diabetic STZ + water-treated group (p ≤ 0.05). C, nondiabetic saline + water control. SH, diabetic STZ + water; SS, diabetic STZ + suramin.
Suramin-Treated STZ Rats Have Decreased Levels of DN Markers.
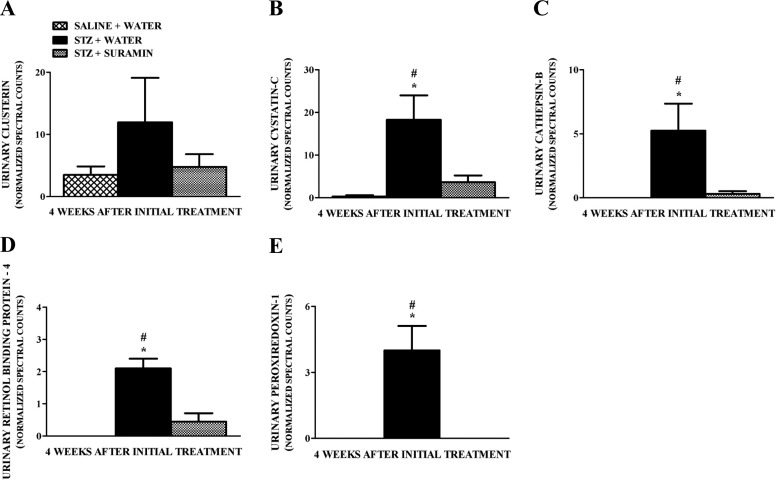
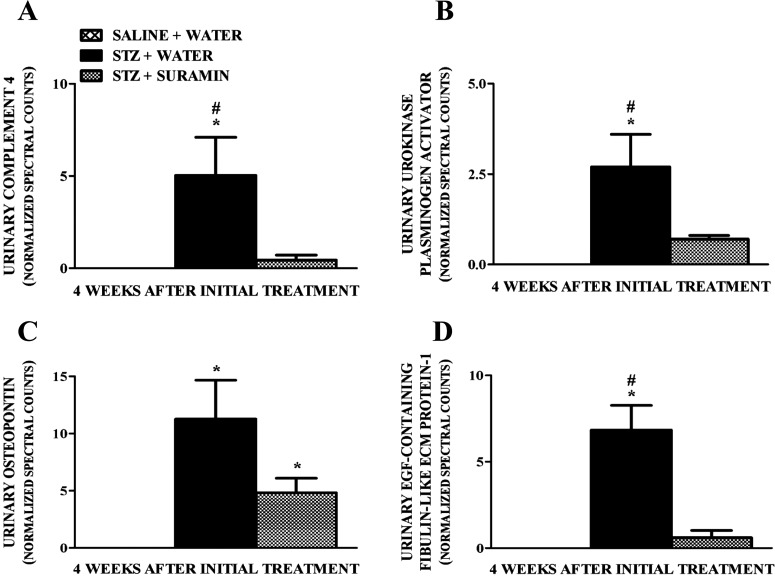
Urinary excretion of biomarkers such as clusterin (Ozer, 2010), cystatin C (Zhang et al., 2010; Jeon et al., 2011), cathepsin B (Meinhardt et al., 2003), retinol-binding protein-4 (Hong and Chia, 1998; Ozer, 2010), and peroxiredoxin-1 (Barati et al., 2007) have been shown to increase in DN. Although we did not observe overt renal damage and dysfunction in our early STZ-induced diabetes model, we detected increased levels of these biomarkers in the urine of STZ rats compared with controls, and suramin blocked the increase in these biomarkers (Fig. 3, A–E). We also identified increased urinary complement components such as complement component 4 (Satoskar et al., 2012) and urinary extracellular matrix (ECM)-related proteins such as urokinase plasminogen activator, osteopontin (OPN), and fibulin-like ECM protein 1 (Tada et al., 1994; Nicholas et al., 2010; Satoskar et al., 2012) in the urine of STZ rats compared with controls, and suramin blocked the increase in these biomarkers (Fig. 4, A–D). These biomarkers were shown previously to be involved in glomerulopathy/glomerulosclerosis associated with DN (Satoskar et al., 2012).
Fig. 3.
Quantitation of normalized spectral counts for clusterin (A), cystatin C (B), cathepsin B (C), retinol-binding protein-4 (D), and peroxiredoxin-1 (E) in the urine samples of nondiabetic and diabetic rats ± suramin intervention at week 4 after the initial treatments. Spectral counts were normalized using HIV envelope protein, which served as the internal standard. Data are expressed as means ± S.E. (n = 4). *, significant from the respective nondiabetic saline + water controls. #, significant from the respective diabetic STZ + suramin-treated group (p ≤ 0.05).
Fig. 4.
Quantitation of normalized spectral counts for complement 4 (A), urokinase plasminogen activator (B), osteopontin (C), and epidermal growth factor-containing fibulin-like ECM protein 1 (D) in the urine samples of nondiabetic and diabetic rats ± suramin intervention at week 4 after initial treatments. Spectral counts were normalized using HIV envelope protein, which served as the internal standard. Data are expressed as means ± S.E. (n = 4). *, significant from the respective nondiabetic saline + water controls. #, significant from respective diabetic STZ + suramin-treated group (p ≤ 0.05).
Suramin Inhibits Activation of Proinflammatory Proteins NF-κB and STAT-3 but Not of ERK1/2 in STZ Rat Kidneys.
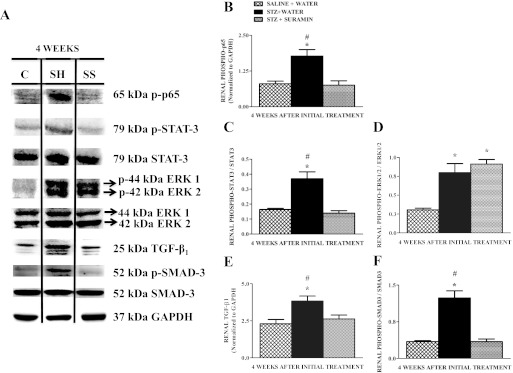
The role of hyperglycemia-induced phosphorylation and activation of proinflammatory mediators such as NF-κB, STAT-3, and ERK1/2 has been established (Soldatos and Cooper, 2008; Kuhad and Chopra, 2009; Ma et al., 2009). Therefore, we tested the hypothesis that suramin might exert its anti-inflammatory effect by modulating these factors in STZ rats. Phosphorylated p65 levels increased in STZ rat kidneys, and this increase was completely blocked by suramin treatment (Fig. 5, A and B). Likewise, phosphorylation of STAT-3 increased 2.5-fold in STZ rat kidneys, and the increase was completely blocked by suramin treatment (Fig. 5, A and C). There were no changes in total STAT-3. Phosphorylation of ERK1/2 also increased 3-fold in STZ rats ± suramin treatment (Fig. 5, A and D).
Fig. 5.
Representative Western blot showing phospho-p65, phospho-STAT-3/total STAT-3, phospho-ERK1/2/total ERK1/2, TGF-β1, and phospho-Smad-3/total Smad-3 protein expressions in kidneys of nondiabetic and diabetic rats ± suramin intervention at week 4 after the initial treatments (A). Densitometric analysis of renal phospho-p65 (B), renal phospho-STAT-3/STAT-3 (C), renal phospho-ERK1/2-ERK1/2 (D), renal TGF-β1 (E), and renal phospho-Smad-3/Smad-3 (F) in kidneys of nondiabetic and diabetic rats ± suramin intervention at week 4 after initial treatments. Data were normalized by GAPDH, which served as the internal control. Data are expressed as means ± S.E. (n = 4). *, significant from the respective nondiabetic saline + water controls. #, significant from the respective diabetic STZ + suramin-treated group (p ≤ 0.05). C, nondiabetic saline + water control. SH, diabetic STZ + water; SS, diabetic STZ + suramin.
Suramin Decreases TGF-β1 and SMAD-3 Signaling.
The pathogenic role of TGF-β1 activation in hyperglycemia and increased accumulation of fibrogenic material during DN has been established (Kanwar et al., 2011). Renal TGF-β1 protein expression was elevated 1.5-fold in STZ rats and suramin treatment completely blocked the increase in TGF-β1 (Fig. 5, A and E). The activated TGF-β1 receptor results in the phosphorylation of SMAD-3 (Rahimi and Leof, 2007; Kanwar et al., 2011). We observed that SMAD-3 phosphorylation in STZ rat kidneys was elevated and that suramin treatment completely blocked the increase in phospho-SMAD-3 (Fig. 5, A and F). Therefore, activation of renal-NF-κB, STAT-3, and TGF-β1 observed in diabetes was blocked by suramin treatment.
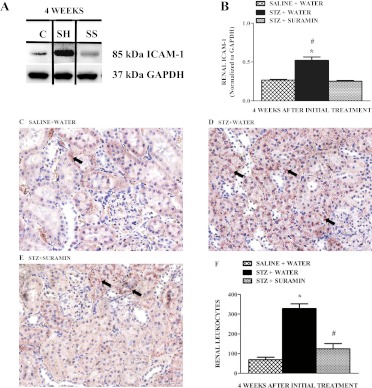
Suramin Attenuates Renal ICAM-1 Expression and Decreases Leukocyte Infiltration in STZ Rat Kidneys.
Activation of renal endothelial cell ICAM-1 and the contributions of leukocytes in the pathophysiology of renal inflammation in DN have been documented previously (Fornoni et al., 2008; Soetikno et al., 2011; Tang et al., 2011). We found that ICAM-1 expression increased 2.5-fold in STZ rats and that suramin completely blocked the increased ICAM-1 expression in STZ rat kidneys (Fig. 6, A and B). We also analyzed leukocyte infiltration in the corticomedullary region of the kidneys by staining neutrophils and monocytes with naphthol AS-D chloroacetate esterase as described previously (Zhuang et al., 2009). Renal leukocyte infiltration increased 4-fold in STZ rats, and suramin treatment decreased leukocyte infiltration to control levels (Fig. 6, C–F).
Fig. 6.
Representative Western blot showing ICAM-1 (A) protein expression and its densitometric analysis (B) in kidneys of nondiabetic and diabetic rats ± suramin intervention at week 4 after the initial treatments. Data were normalized by GAPDH, which served as internal control. Data are expressed as means ± S.E. (n = 4). *, significant from respective nondiabetic saline + water controls; #, significant from respective diabetic STZ + suramin-treated group; p ≤ 0.05. Representative photomicrographs of neutrophil and monocyte staining assessed by the formation of a stable pinkish-red colored (arrows) complex of free naphthol and diazonium salts after incubation of kidney sections from nondiabetic rats (C) and diabetic rats treated without suramin (D) and with suramin (E) after 4 weeks. All fields were chosen from the OSOM. Original magnification, 200×. F, quantitative analysis of renal leukocyte infiltration assessed by the number of pink colored dots in a total of 25 fields in the OSOM region of kidney sections. Data are expressed as means ± S.E. (n = 4). *, significant from the respective nondiabetic saline + water controls. #, significant from the respective diabetic STZ + water-treated group (p ≤ 0.05). C, nondiabetic saline + water control. SH, diabetic STZ + water; SS, diabetic STZ + suramin.
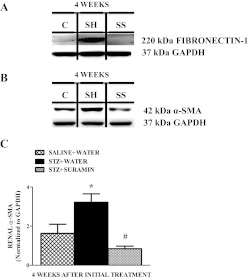
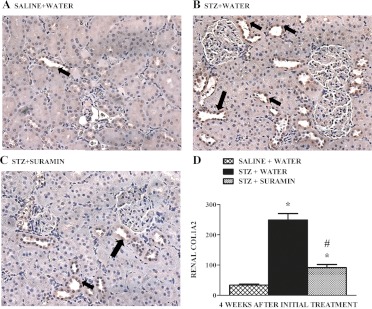
Suramin Decreases TGF-β1/SMAD-3-Activated Fibrogenic Mediators and Fibrogenesis in STZ Rat Kidneys.
The TGF-β1-phospho-Smad-3 complex is known to translocate into the nucleus and regulate transcription of TGF-β1/SMAD-3-activated fibrogenic genes such as fibronectin-1, α-SMA, and collagen 1A2 (Rahimi and Leof, 2007; Kanwar et al., 2011). Renal fibronectin-1 and α-SMA protein expression increased in STZ rats, and suramin completely blocked these increases (Fig. 7). Kidneys from STZ rats exhibited a 2.5-fold increase in COL1A2 protein expression in the tubulointerstitium compared with that in control rats 4 weeks after STZ treatment, and suramin treatment markedly decreased, but did not completely block, the expression of COL1A2 (Fig. 8). To test whether a lower dose of suramin was effective in preventing fibrogenesis in STZ rat kidneys, we examined expression of renal TGF-β1 protein (data not shown) and its target fibrogenic markers fibronectin-1 and α-SMA protein expression (data not shown) and found that 5 mg/kg did not block the expression of these fibrogenic markers compared with 10 mg/kg suramin.
Fig. 7.
Representative Western blot showing fibronectin-1 (A) and α-SMA (B) protein expressions in kidneys of nondiabetic and diabetic rats ± suramin intervention at week 4 after the initial treatments. C, densitometric analysis of renal α-SMA protein in kidneys of nondiabetic and diabetic rats ± suramin intervention at week 4 after the initial treatments. Data were normalized by GAPDH, which served as the internal control. Data are expressed as means ± S.E. (n = 4). *, significant from the respective nondiabetic saline + water controls (p ≤ 0.05). #, significant from the respective diabetic STZ + water-treated group (p ≤ 0.05). C, nondiabetic saline + water control. SH, diabetic STZ + water; SS, diabetic STZ + suramin.
Fig. 8.
Representative photomicrographs of COL1A2 expression in kidney sections from nondiabetic rats (A) and diabetic rats treated without suramin (B) and with suramin (C) after 4 weeks. All fields were chosen from the OSOM. Original magnification, 200×. D, quantitative analysis of renal COL1A2 assessed by brown staining in the epithelial cells lining the proximal tubules (arrows) in a total of 25 fields in the OSOM region of kidney sections. Data are expressed as mean ± S.E. (n = 4). *, significant from respective nondiabetic saline + water controls. #, significant from the respective diabetic STZ + water-treated group (p ≤ 0.05).
Discussion
We chose the STZ rat model and the 4-week time point for these studies because of our interest in the early events in diabetic renal injury and because it is difficult to study signaling pathways in the presence of overt histopathological changes. We did not observe histopathological changes among nondiabetic and diabetic rats at this time point.
In this current model of early hyperglycemia-induced renal injury, we observed marked differences in urinary biomarkers of early DN, proinflammatory mediators and profibrotic proteins in diabetic rat kidneys. Urinary cystatin C, which was elevated in STZ rats, is an indicator of glomerular dysfunction (Ozer, 2010). Increased urinary excretion of retinol binding protein-4 has been reported in diabetic patients, and elevated levels of this biomarker in urine is an indicator of tubular dysfunction (Shimizu et al., 1992). Cathepsin B, a mitochondrial and lysosomal enzyme, is a biomarker for DN (Meinhardt et al., 2003). Even though urinary clusterin was not increased statistically, because of high variability, it is an early indicator of tubular and glomerular dysfunction (Ozer, 2010). Increased antioxidative enzyme peroxiredoxin-1 in the urine of STZ rats suggests an adaptive response to oxidative stress associated with this diabetic model (Barati et al., 2007). Of interest, excretion of all these urinary markers in rats was markedly elevated 4 weeks after STZ. More importantly, suramin intervention reversed these effects. Therefore, with the development of suramin as a treatment for DN, these urinary biomarkers can serve to identify treatment effects.
The observation that hyperglycemia did not induce any tubular damage in this early model of diabetes is consistent with the observation that renal KIM-1 did not increase. Only a limited number of studies to date have investigated the association of tubular markers such as KIM-1 with the severity of chronic kidney disease in DN. In these studies, increased urinary KIM-1 correlated with diabetes (Nauta et al., 2011). However, we did not observe any mass spectra related to KIM-1 in the urinalysis by LC-MS/MS. NGAL has been previously shown to play an important role in the progression of DN (Bolignano et al., 2009a; Lalanne et al., 2011; Nauta et al., 2011; Nielsen et al., 2011). It has also been reported that NGAL may play a role in renal adaptation to diabetes, probably as a defensive mechanism to mitigate tubular injury (Bolignano et al., 2009b). NGAL has also been reported to have antioxidant properties (Roudkenar et al., 2007, 2008, 2011; Bahmani et al., 2010). Of interest, in our study, renal NGAL increased in diabetic rats, and suramin intervention further increased renal NGAL. Although the mechanism and importance of this effect are not known, our finding that suramin mediated the increase in renal NGAL in STZ rats is consistent with our recent report in a model of glycerol-induced acute kidney injury, in which delayed administration of suramin up-regulates NGAL in rat kidneys (Korrapati et al., 2012).
We also did not see any noticeable changes in glomerular pathology in this early diabetes model. However, we identified increased urinary excretion of complement component 4 and fibulin-like ECM protein 1, which are known to be involved in the development of fibronectin-mediated glomerulopathy (Østergaard et al., 2005; Satoskar et al., 2012). Increased expression and activity of urokinase plasminogen activator in mesangial cells of diabetic kidneys leading to increased proteolysis of mesangial matrix and development of diabetic glomerulosclerosis was shown previously (Tada et al., 1994). In addition, the profibrotic adhesion molecule OPN was shown to enhance glomerular damage in DN mouse models, probably through the TGF-β1 pathway, whereas deletion of OPN protected against progression of DN (Nicholas et al., 2010). Whereas the mechanism by which suramin reverses the appearance of all these proteins in STZ rat urine is not known, it is possible that it is the result of suramin blocking TGF-β1 signaling (Nicholas et al., 2010; Kocic et al., 2012). Thus, the application of LC-MS/MS techniques may provide crucial insights into tubular and glomerular changes that cannot be revealed by using traditional renal functional and pathological parameters and also highlight potential pathogenic mechanisms not previously known or considered for DN.
Increased nuclear translocation of NF-κB (phosphorylation of p65 subunit) in endothelial cells caused by glucotoxicity has been linked to transcriptional activation of adhesion molecules such as ICAM-1 (Ohga et al., 2007). Circulating leukocytes use endothelial cell transmembrane ICAM-1 as an anchor to transmigrate and cause inflammation (Nayak, 2005). Activation of renal endothelial cell ICAM-1 expression by hyperglycemia along with subsequent leukocyte infiltration has been suggested to be an important event in the pathophysiology of tubular inflammation in DN (Fornoni et al., 2008; Soetikno et al., 2011; Wu et al., 2011; Lin et al., 2012). We observed that suramin intervention after induction of diabetes decreased phosphorylation of p65, blocked ICAM-1 expression, and decreased leukocyte infiltration. However, mechanistically these sequential events may result from a direct inhibitory effect of suramin on NF-κB activation as reported previously (Goto et al., 2006).
High-glucose is also known to activate transforming growth factor-β1, which is widely thought to be the cytokine most pertinent to the ECM-related renal pathology typically observed in patients with chronic progressive DN (Kanwar et al., 2011). When stimulated, TGF-β1 activates the TGF-β1 receptor, SMAD-3 is phosphorylated, and the resulting complex translocates into the nucleus and regulates transcription of TGF-β1 target fibrogenic genes such as collagen 1A2 and fibronectin-1 (Rahimi and Leof, 2007). It has been shown previously, that administration of neutralizing anti-TGF-β1 antibodies prevents renal hypertrophy, mesangial matrix expansion, increases in collagen and fibronectin expression, and declines in renal function in mice with STZ-induced diabetes, leaving little doubt that this profibrogenic cytokine is intricately involved in the renal pathobiology of diabetes (Sharma et al., 1996; Song et al., 2006).
Suramin was previously suggested to inhibit fibrotic and growth inhibitory actions of TGF-β1 and inhibit renal fibrosis (Liu and Zhuang, 2011; Liu et al., 2011b; Korrapati et al., 2012). In our study, we found that delayed administration of suramin after the induction of diabetes inhibited renal TGF-β1 expression and subsequent SMAD-3 activation in diabetic rats. Because α-SMA-positive myofibroblasts are the principal effector cells responsible for ECM overproduction in the fibrotic kidney along with other critical profibrotic proteins such as fibronetin-1 and collagen 1A2 (Wynn, 2008), suramin largely reduced expression of α-SMA, blocked expression of fibronectin-1, and decreased deposition of COL1A2 in the tubular epithelial cells. Collectively, we suggest that suramin acts upstream of TGF-β1-SMAD-3 signaling and blocks this pathway after a significant degree of early DN-induced tubulointerstitial fibrosis has occurred with decreases in TGF-β1-SMAD-3-targeted fibrogenic proteins fibronectin, α-SMA, and collagen 1A2 in diabetic rats.
Suramin is known to accumulate in the kidney, its plasma half-life is 44 to 54 days, and its total body clearance is very low (0.41 ml/min) (Collins et al., 1986; Liu and Zhuang, 2011). These pharmacokinetic data show that suramin has sustained effects and suggest that weekly dosing or longer would be effective in treating diabetes-induced renal injury.
It has been well documented that activation of NF-κB is a major contributor in inflammation associated with DN (Fornoni et al., 2008; Soetikno et al., 2011). Hyperglycemia is also known to induce activation of mitogen-activated protein kinases (e.g., ERK1/2), the STAT-3 pathway, and/or G protein-mediated aberrant expression of ECM proteins (Marrero et al., 2006; Catania et al., 2007; Tan et al., 2007). These kinases participate in transcriptional up-regulation of collagen and fibronectin in DN (Suzuki et al., 2004; Pang et al., 2010; Liu et al., 2011b). Phosphorylation and subsequent activation of NF-κB and STAT-3 were blocked by suramin in diabetic rat kidneys. However, we did not observe a difference in ERK1/2 activation in diabetic rat kidneys with or without suramin intervention, suggesting that the effect of suramin is at the transcriptional level in this model. Because the STAT-3 pathway is activated in response to a variety of growth factor/cytokine receptors (Ivashkiv and Hu, 2004), inhibition of this signaling by suramin may be due to inactivation of some membrane receptors such as TGF-β1. These data, together with suramin-mediated blockade of SMAD-3 activation suggest that suramin administration is able to inhibit multiple signaling pathways that contribute to renal fibrogenesis after DN and thereby block the progression of DN.
In conclusion, this work advances the field and is translational by demonstrating a potential therapy for diabetic nephropathy, by showing approximate sites of actions of suramin, and by identifying urinary markers of diabetic nephropathy that change in response to suramin treatment.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM084147]; the National Institutes of Health National Center for Research Resources [Grant UL1-RR029882]; the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs; the South Carolina Clinical and Translational Research Institute, with an academic home at the Medical University of South Carolina. Animal facilities were funded by National Institutes of Health National Center for Research Resources [Grant C06-RR015455].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- DN
- diabetic nephropathy
- ESRD
- end-stage renal disease
- STZ
- streptozotocin
- LC
- liquid chromatography
- MS/MS
- tandem mass spectrometry
- COL1A2
- collagen 1A2
- KIM-1
- kidney injury molecule-1
- NGAL
- neutrophil gelatinase-associated lipocalin-2
- TGF-β1
- transforming growth factor-β1
- α-SMA
- smooth muscle actin
- ICAM-1
- intracellular adhesion molecule-1
- phospho
- phosphorylated
- ERK1/2
- extracellular regulated kinase 1/2
- STAT-3
- signal transducer and activator of transcription factor-3
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- SPE
- solid-phase extraction
- OSOM
- outer stripe of the outer medulla
- ECM
- extracellular matrix
- OPN
- osteopontin.
Authorship Contributions
Participated in research design: Korrapati and Schnellmann.
Conducted experiments: Korrapati, Shaner, Neely, and Alge.
Contributed new reagents or analytic tools: Neely, Alge, and Arthur.
Performed data analysis: Korrapati, Shaner, Neely, Alge, and Schnellmann.
Wrote or contributed to the writing of the manuscript: Korrapati, Neely, Alge, Arthur, and Schnellmann.
References
- Bahmani P, Halabian R, Rouhbakhsh M, Roushandeh AM, Masroori N, Ebrahimi M, Samadikuchaksaraei A, Shokrgozar MA, Roudkenar MH. (2010) Neutrophil gelatinase-associated lipocalin induces the expression of heme oxygenase-1 and superoxide dismutase 1, 2. Cell Stress Chaperones 15:395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakumar P, Arora MK, Ganti SS, Reddy J, Singh M. (2009) Recent advances in pharmacotherapy for diabetic nephropathy: current perspectives and future directions. Pharmacol Res 60:24–32 [DOI] [PubMed] [Google Scholar]
- Barati MT, Merchant ML, Kain AB, Jevans AW, McLeish KR, Klein JB. (2007) Proteomic analysis defines altered cellular redox pathways and advanced glycation end-product metabolism in glomeruli of db/db diabetic mice. Am J Physiol Renal Physiol 293:F1157–F1165 [DOI] [PubMed] [Google Scholar]
- Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M. (2009a) Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 4:337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolignano D, Lacquaniti A, Coppolino G, Donato V, Fazio MR, Nicocia G, Buemi M. (2009b) Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press Res 32:91–98 [DOI] [PubMed] [Google Scholar]
- Catania JM, Chen G, Parrish AR. (2007) Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol Renal Physiol 292:F905–F911 [DOI] [PubMed] [Google Scholar]
- Collins JM, Klecker RW, Jr, Yarchoan R, Lane HC, Fauci AS, Redfield RR, Broder S, Myers CE. (1986) Clinical pharmacokinetics of suramin in patients with HTLV-III/LAV infection. J Clin Pharmacol 26:22–26 [DOI] [PubMed] [Google Scholar]
- Covington MD, Schnellmann RG. (2012) Chronic high glucose downregulates mitochondrial calpain 10 and contributes to renal cell death and diabetes-induced renal injury. Kidney Int 81:391–400 [DOI] [PubMed] [Google Scholar]
- Fornoni A, Ijaz A, Tejada T, Lenz O. (2008) Role of inflammation in diabetic nephropathy. Curr Diabetes Rev 4:10–17 [DOI] [PubMed] [Google Scholar]
- Goto T, Takeuchi S, Miura K, Ohshima S, Mikami K, Yoneyama K, Sato M, Shibuya T, Watanabe D, Kataoka E, et al. (2006) Suramin prevents fulminant hepatic failure resulting in reduction of lethality through the suppression of NF-κB activity. Cytokine 33:28–35 [DOI] [PubMed] [Google Scholar]
- Hong CY, Chia KS. (1998) Markers of diabetic nephropathy. J Diabetes Complications 12:43–60 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Ivashkiv LB, Hu X. (2004) Signaling by STATs. Arthritis Res Ther 6:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YK, Kim MR, Huh JE, Mok JY, Song SH, Kim SS, Kim BH, Lee SH, Kim YK, Kim IJ. (2011) Cystatin C as an early biomarker of nephropathy in patients with type 2 diabetes. J Korean Med Sci 26:258–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar YS, Sun L, Xie P, Liu FY, Chen S. (2011) A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol 6:395–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74:5383–5392 [DOI] [PubMed] [Google Scholar]
- Kocic J, Bugarski D, Santibanez JF. (2012) SMAD3 is essential for transforming growth factor-β1-induced urokinase type plasminogen activator expression and migration in transformed keratinocytes. Eur J Cancer 48:1550–1557 [DOI] [PubMed] [Google Scholar]
- Korrapati MC, Shaner BE, Schnellmann RG. (2012) Recovery from glycerol-induced acute kidney injury is accelerated by suramin. J Pharmacol Exp Ther 341:126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhad A, Chopra K. (2009) Attenuation of diabetic nephropathy by tocotrienol: involvement of NFκB signaling pathway. Life Sci 84:296–301 [DOI] [PubMed] [Google Scholar]
- Lalanne A, Beaudeux JL, Bernard MA. (2011) NGAL: a biomarker of acute and chronic renal dysfunction. Ann Biol Clin 69:629–636 [DOI] [PubMed] [Google Scholar]
- Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, Au WS, Chan KW, Lai KN, Tang SC. (2012) Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol 23: 86–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, He S, Tolbert E, Gong R, Bayliss G, Zhuang S. (2012) Suramin alleviates glomerular injury and inflammation in the remnant kidney. PLoS One 7:e36194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Tolbert E, Pang M, Ponnusamy M, Yan H, Zhuang S. (2011a) Suramin inhibits renal fibrosis in chronic kidney disease. J Am Soc Nephrol 22:1064–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Tolbert E, Ponnusamy M, Yan H, Zhuang S. (2011b) Delayed administration of suramin attenuates the progression of renal fibrosis in obstructive nephropathy. J Pharmacol Exp Ther 338:758–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Zhuang S. (2011) Tissue protective and anti-fibrotic actions of suramin: new uses of an old drug. Curr Clin Pharmacol 6:137–142 [DOI] [PubMed] [Google Scholar]
- Ma FY, Liu J, Nikolic-Paterson DJ. (2009) The role of stress-activated protein kinase signaling in renal pathophysiology. Braz J Med Biol Res 42:29–37 [DOI] [PubMed] [Google Scholar]
- Marrero MB, Banes-Berceli AK, Stern DM, Eaton DC. (2006) Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am J Physiol Renal Physiol 290:F762–F768 [DOI] [PubMed] [Google Scholar]
- Mathew A, Cunard R, Sharma K. (2011) Antifibrotic treatment and other new strategies for improving renal outcomes. Contrib Nephrol 170:217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt U, Ammann RA, Flück C, Diem P, Mullis PE. (2003) Microalbuminuria in diabetes mellitus: efficacy of a new screening method in comparison with timed overnight urine collection. J Diabetes Complications 17:254–257 [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. (2003) Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289:76–79 [DOI] [PubMed] [Google Scholar]
- Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW. (2004) Nephropathy in diabetes. Diabetes Care 27 (Suppl 1):S79–S83 [DOI] [PubMed] [Google Scholar]
- Najafian B, Alpers CE, Fogo AB. (2011) Pathology of human diabetic nephropathy. Contrib Nephrol 170:36–47 [DOI] [PubMed] [Google Scholar]
- Nauta FL, Boertien WE, Bakker SJ, van Goor H, van Oeveren W, de Jong PE, Bilo H, Gansevoort RT. (2011) Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care 34:975–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak BK. (2005) Changing times, evolving responses. Indian J Ophthalmol 53:1–3 [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R. (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75:4646–4658 [DOI] [PubMed] [Google Scholar]
- Nicholas SB, Liu J, Kim J, Ren Y, Collins AR, Nguyen L, Hsueh WA. (2010) Critical role for osteopontin in diabetic nephropathy. Kidney Int 77:588–600 [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Hansen HP, Jensen BR, Parving HH, Rossing P. (2011) Urinary neutrophil gelatinase-associated lipocalin and progression of diabetic nephropathy in type 1 diabetic patients in a four-year follow-up study. Nephron Clin Pract 118:c130–135 [DOI] [PubMed] [Google Scholar]
- Ohga S, Shikata K, Yozai K, Okada S, Ogawa D, Usui H, Wada J, Shikata Y, Makino H. (2007) Thiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-κB activation. Am J Physiol Renal Physiol 292:F1141–F1150 [DOI] [PubMed] [Google Scholar]
- Østergaard J, Hansen TK, Thiel S, Flyvbjerg A. (2005) Complement activation and diabetic vascular complications. Clin Chim Acta 361:10–19 [DOI] [PubMed] [Google Scholar]
- Ozer JS. (2010) A guidance for renal biomarker lead optimization and use in translational pharmacodynamics. Drug Discov Today 15:142–147 [DOI] [PubMed] [Google Scholar]
- Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, Chin YE, Yan H, Dworkin LD, Zhuang S. (2010) A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int 78:257–268 [DOI] [PubMed] [Google Scholar]
- Rahimi RA, Leof EB. (2007) TGF-β signaling: a tale of two responses. J Cell Biochem 102:593–608 [DOI] [PubMed] [Google Scholar]
- Roudkenar MH, Halabian R, Bahmani P, Roushandeh AM, Kuwahara Y, Fukumoto M. (2011) Neutrophil gelatinase-associated lipocalin: a new antioxidant that exerts its cytoprotective effect independent on heme oxygenase-1. Free Radic Res 45:810–819 [DOI] [PubMed] [Google Scholar]
- Roudkenar MH, Halabian R, Ghasemipour Z, Roushandeh AM, Rouhbakhsh M, Nekogoftar M, Kuwahara Y, Fukumoto M, Shokrgozar MA. (2008) Neutrophil gelatinase-associated lipocalin acts as a protective factor against H2O2 toxicity. Arch Med Res 39:560–566 [DOI] [PubMed] [Google Scholar]
- Roudkenar MH, Kuwahara Y, Baba T, Roushandeh AM, Ebishima S, Abe S, Ohkubo Y, Fukumoto M. (2007) Oxidative stress induced lipocalin 2 gene expression: addressing its expression under the harmful conditions. J Radiat Res 48:39–44 [DOI] [PubMed] [Google Scholar]
- Satoskar AA, Shapiro JP, Bott CN, Song H, Nadasdy GM, Brodsky SV, Hebert LA, Birmingham DJ, Nadasdy T, Freitas MA, et al. (2012) Characterization of glomerular diseases using proteomic analysis of laser capture microdissected glomeruli. Mod Pathol 25:709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B, Balomajumder C, Roy P. (2008) Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food Chem Toxicol 46:2376–2383 [DOI] [PubMed] [Google Scholar]
- Sharma K, Jin Y, Guo J, Ziyadeh FN. (1996) Neutralization of TGF-β by anti-TGF-β antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 45:522–530 [DOI] [PubMed] [Google Scholar]
- Shimizu H, Negishi M, Shimomura Y, Mori M. (1992) Changes in urinary retinol binding protein excretion and other indices of renal tubular damage in patients with non-insulin dependent diabetes. Diabetes Res Clin Pract 18:207–210 [DOI] [PubMed] [Google Scholar]
- Soetikno V, Sari FR, Veeraveedu PT, Thandavarayan RA, Harima M, Sukumaran V, Lakshmanan AP, Suzuki K, Kawachi H, Watanabe K. (2011) Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab (Lond) 8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldatos G, Cooper ME. (2008) Diabetic nephropathy: important pathophysiologic mechanisms. Diabetes Res Clin Pract 82 (Suppl 1):S75–S79 [DOI] [PubMed] [Google Scholar]
- Song JH, Cha SH, Lee HJ, Lee SW, Park GH, Lee SW, Kim MJ. (2006) Effect of low-dose dual blockade of renin-angiotensin system on urinary TGF-β in type 2 diabetic patients with advanced kidney disease. Nephrol Dial Transplant 21:683–689 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Uchida K, Nitta K, Nihei H. (2004) Role of mitogen-activated protein kinase in the regulation of transforming growth factor-β-induced fibronectin accumulation in cultured renal interstitial fibroblasts. Clin Exp Nephrol 8:188–195 [DOI] [PubMed] [Google Scholar]
- Tada H, Tsukamoto M, Ishii H, Isogai S. (1994) A high concentration of glucose alters the production of tPA, uPA and PAI-1 antigens from human mesangial cells. Diabetes Res Clin Pract 24:33–39 [DOI] [PubMed] [Google Scholar]
- Tan AL, Forbes JM, Cooper ME. (2007) AGE, RAGE, and ROS in diabetic nephropathy. Semin Nephrol 27:130–143 [DOI] [PubMed] [Google Scholar]
- Tang SC, Leung JC, Lai KN. (2011) Diabetic tubulopathy: an emerging entity. Contrib Nephrol 170:124–134 [DOI] [PubMed] [Google Scholar]
- Wang GG, Lu XH, Li W, Zhao X, Zhang C. (2011) Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid Based Complement Alternat Med 2011:323171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Sytwu HK, Lu KC, Lin YF. (2011) Role of T cells in type 2 diabetic nephropathy. Exp Diabetes Res 2011:514738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PP, Zhan JF, Xie HL, Li LS, Liu ZH. (2010) Evaluation of glomerular filtration rate using cystatin C in diabetic patients analysed by multiple factors including tubular function. J Int Med Res 38:473–483 [DOI] [PubMed] [Google Scholar]
- Zhuang S, Lu B, Daubert RA, Chavin KD, Wang L, Schnellmann RG. (2009) Suramin promotes recovery from renal ischemia/reperfusion injury in mice. Kidney Int 75:304–311 [DOI] [PubMed] [Google Scholar]