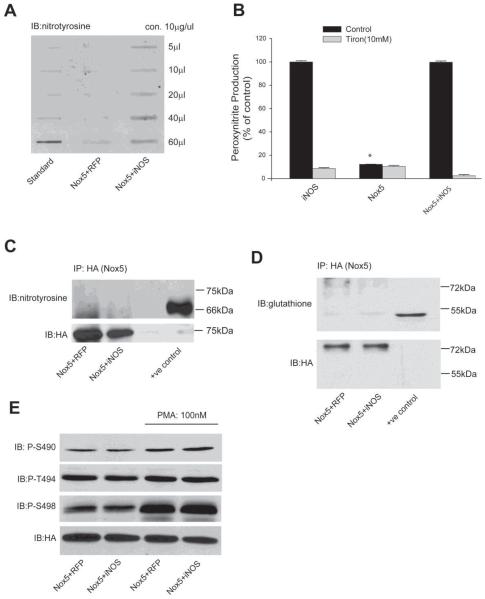
Figure 6. Endogenously produced NO does not alter the nitration, phosphorylation or glutathiolation of Nox5.
(A) COS-7 cells were co-transfected with Nox5 and either iNOS or RFP, and 48h post-transfection, total protein nitration was measured via slot blot and anti-nitrotyrosine immunoblotting. (B) Peroxynitrite (ONOO−) was measured by DHR 123 fluorescence in the presence or absence of the antioxidant Tiron (10mM). Results are presented as % of control (means ± S.E., n =6 * p<0.05 versus iNOS). (C-D) Nox5 was immunoprecipitated from COS-7 cells co-transfected with iNOS or RFP and immunoblotted for nitrotyrosine (C) or glutathione (D). Nitrated BSA (bovine serum albumin) was used as a positive control for nitrotyrosine and oxidized actin from HeLa cell extracts for glutathiolated proteins (Virogen). (E) Nox5 was co-transfected with iNOS or RFP and 48h after transfection, cells were treated with vehicle or PMA (100nM) for 30mins, and the phosphorylation of Nox5 on S490, T494 and S498 was detected by phosphorylation state specific antibodies. Total Nox5 expression was detected using anti-HA antibody. Results are representative of at least 3 separate experiments.