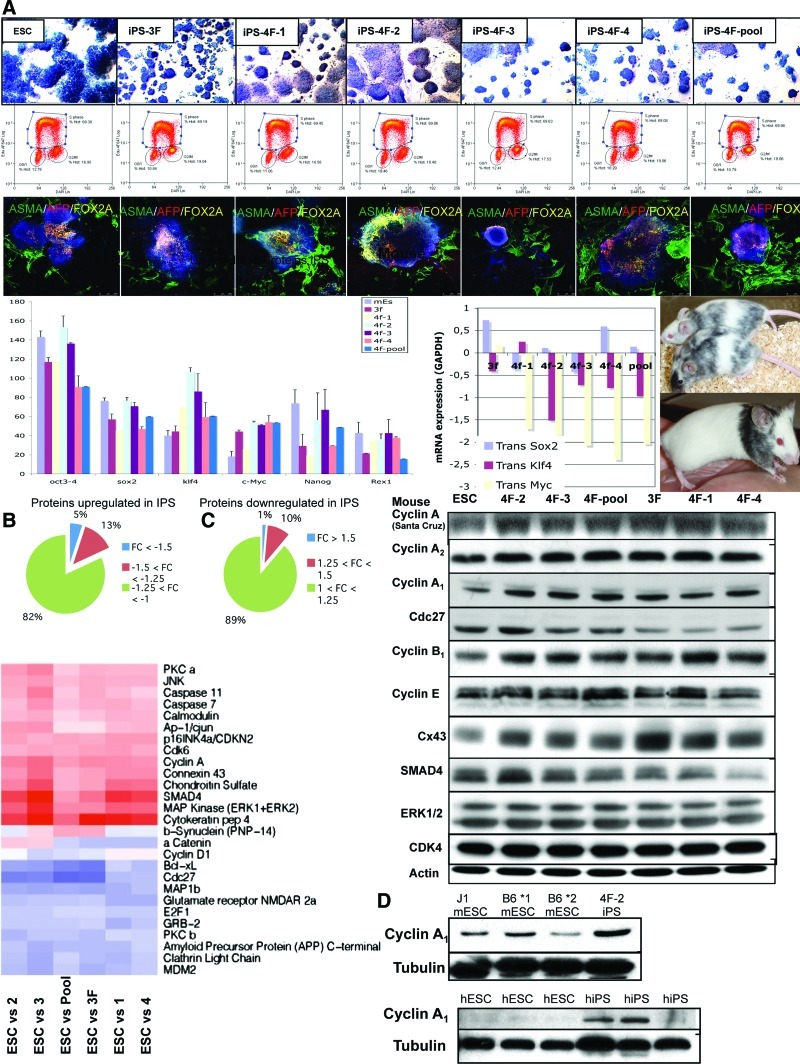
FIG. 1.
Characterization of mouse embryonic stem (ES) and induced pluripotent stem (iPS) cells. (A) Top 3 sets of panels: AP staining of C57/BL6 mouse ES cell (mESC) lines (2 lines tested, data not shown for both) and mouse iPS (miPS) cells (3F: 3 factors; 4F: 4 factors) made from MEFs of C57/BL6 mice (×100). 4F-pool is a pool of 4 iPS clones. Cell cycle analysis by fluorescence-activated cell sorting (FACS) for EDU (BRDU) in each clone demonstrates a high percentage of cells in the S phase in all cases. Note that iPS cells have a higher level of cells in the G2/M phase compared to mESCs. Confocal microscopy photos of EBs differentiating into ectoderm, endoderm, and endoderm demonstrating that mESCs can form all 3 germ layers, but some iPS clones formed low levels of ectoderm. See Supplementary Fig. S1 for further stainings. Lower panels: Graph of real-time polymerase chain reaction (RT-PCR) of endogenous and transgene pluripotency gene expression in ESCs and iPS cells. Transgene expression was almost silenced in iPS cell clones. Photos of chimera mice made from mESCs (top) and miPS cells (bottom). (B) Proteomic analyses using antibody arrays reveal differences between ES and iPS, implicating cyclin A1. Pie graph of differences in protein expression between mESCs and miPS cells and a heat map of antibody array showing changes in protein expression between ESCs and iPS cells. (C) Western blot validation of some of the proteins identified from the antibody array with the greatest changes. (D) Western blot in 3 mESC lines compared to the 1 miPS cell line (4F-2 from C above) for cyclin A1 expression levels to validate lower expression levels of cyclin A1 in mESC lines. Lower panel demonstrates western blot for 3 human ESC (hESC) lines and 3 human iPS (hiPS) cell lines, showing higher levels of cyclin A1 in the hiPS cell lines. AP, alkaline phosphatase; MEFs, mouse embryonic fibroblasts. Color images available online at www.liebertonline.com/scd