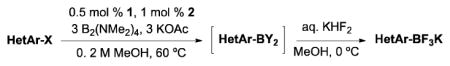
Table 2.
Trifluoroborate Synthesis from Heteroaryl Chlorides and Bromides
| ||||
|---|---|---|---|---|
| entry | X | product | time (h) | % isolated Yield |
| 1 | Cl |
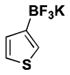
|
6 | 45 |
| 2 | Cl |
|
5 | 5 |
| 3 | Cl |
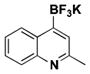
|
11 | 58, 47a |
| 4 | Br |
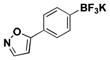
|
5.5 | 37, 85a |
| 5 | Br |
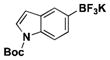
|
4.5 | 96, 93a |
| 6 | Br |
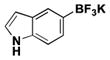
|
6 | 70 |
| 7 | Cl |
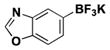
|
5 | 36, 81a |
| 8 | Br |
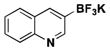
|
5 | 64, 68a |
General conditions: 0.5 mol % of 1, 1.0 mol % of 2, 3.0 equiv of KOAc, 3.0 equiv of B2(NMe2)4, MeOH (0.2 M), 60 °C for time indicated.
Yield from previous method with B2(OH)4 as boron source (ref 15).
