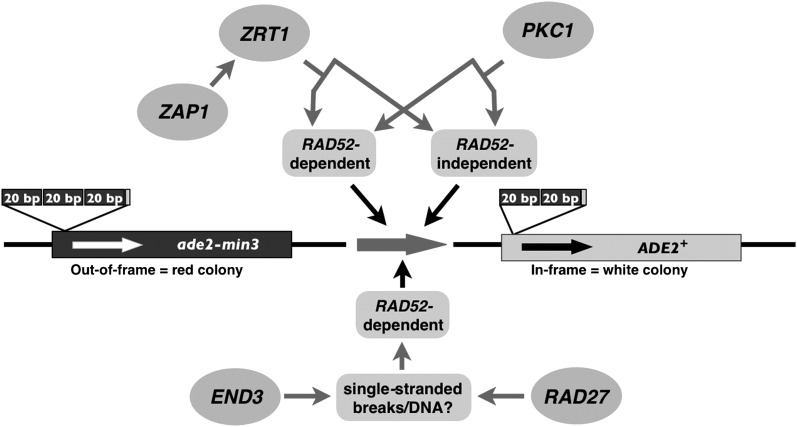
Figure 4.
Model for pathways regulating ade2-min3 minisatellite stability during stationary phase. Loss of components in multiple pathways can lead to ade2-min3 minisatellite alterations. In the first pathway, loss of ZRT1 or ZAP1 stimulates loss of one ade2-min3 repeat unit via both RAD52-dependent and RAD52-independent recombination (Kelly et al. 2007). In a second pathway, mutation of PKC1 stimulates loss of one ade2-min3 repeat unit via both RAD52-dependent and RAD52-independent recombination. A third independent pathway is represented by END3, loss of which stimulates deletion of one ade2-min3 repeat unit via RAD52-dependent recombination. This deletion may be due to formation of ssDNA as a consequence of oxidative DNA damage. Alternatively, END3 could be acting in the RAD52-dependent portions of the ZRT1 and PKC1 pathways (not shown). In the final pathway, loss of RAD27 stimulates both loss of one and gain of two ade2-min3 repeats. These minisatellite alterations may be due to formation of ssDNA when RAD27-dependent DNA flap removal does not occur properly. RAD27-dependent minisatellite alterations during mitotic growth require RAD52, so it is possible that stationary phase minisatellite alterations in this pathway also occur via RAD52-dependent recombination.