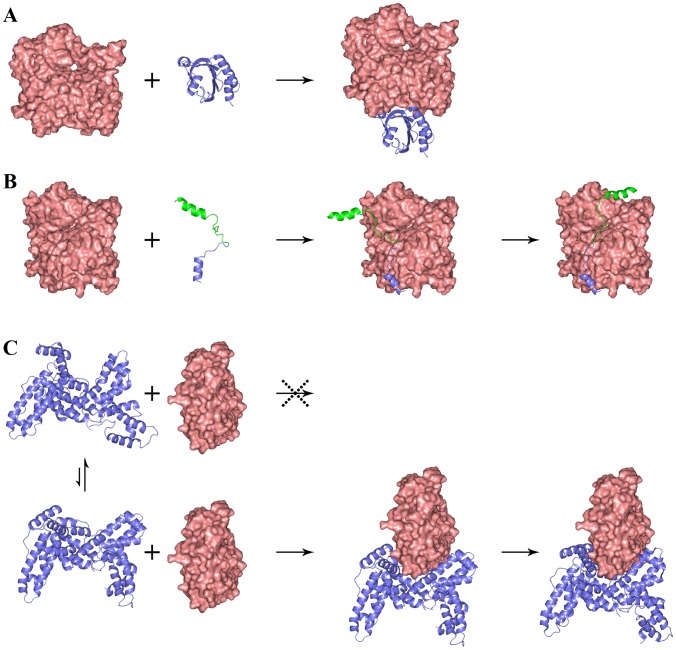
Figure 3. Three classes of association mechanisms.
(A) A relatively rigid globular ABP like profilin reaches the transient complex (not shown) with G-actin by diffusion and then forms the contacts nearly all at once to produce the native complex. The binding of twinfilin ADF-homology domain 2 and gelsolin domain 1 to G-actin follows the same mechanism. (B) The dock-and-coalesce mechanism for the G-actin binding of WASP WCA, an intrinsically disordered protein. (C) Vitamin-D binding protein has an opening between its domains 1 and 3, in both the free state and the G-actin-bound state, that is too narrow for G-actin to enter. So DBP must undergo breathing motion to transiently widen its opening. Once G-actin is inside, the opening quickly narrows to clamp around G-actin. In (A) G-actin has the orientation as in Figure 1; in (B) it is rotated clockwise (viewed from top) around a vertical axis by 45°; and in (C) it is rotated counterclockwise (viewed from top) around a vertical axis by 90°.