Figure 4. Formation of ternary complex between TEP1*S1cut and LRIM1/APL1C.
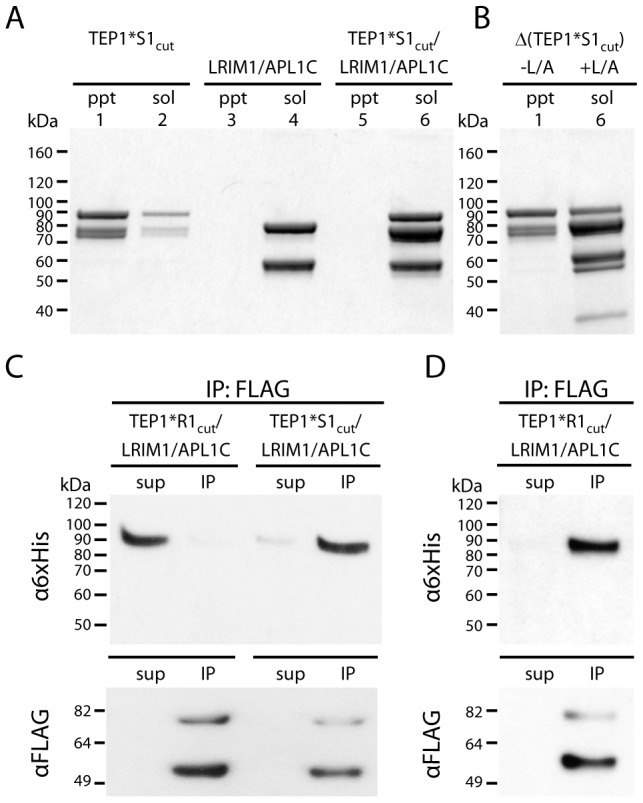
(A) Insoluble (ppt) and soluble (sol) fractions of TEP1*S1cut (lanes 1–2), LRIM1/APL1C (3–4) and TEP1*S1cut/LRIM1/APL1C (5–6) after 36 h at 20°C. (B) Autolytic cleavage assay (Δ) shows the thioester bond in insoluble TEP1*S1cut (1) is hydrolyzed while soluble TEP1*S1cut in complex with LRIM1/APL1C (6) has an intact thioester (heat-induced cleavage of TEP1 C-terminal 85 kDa band). (C) FLAG co-immunoprecipitation of TEP1*R1cut and TEP1*S1cut with LRIM1/APL1C after 48 h (TEP1*R1cut) and 24 h (TEP1*S1cut), and (D) after 24 days (TEP1*R1cut). Proteins in both the supernatant (sup) and immunoprecipitated (IP) samples were detected with Western blotting using either α6×His antibody to detect APL1C-6×His and TEP1-6×His C-terminal chain, or αFLAG antibody to detect LRIM1-FLAG/APL1C-FLAG.
