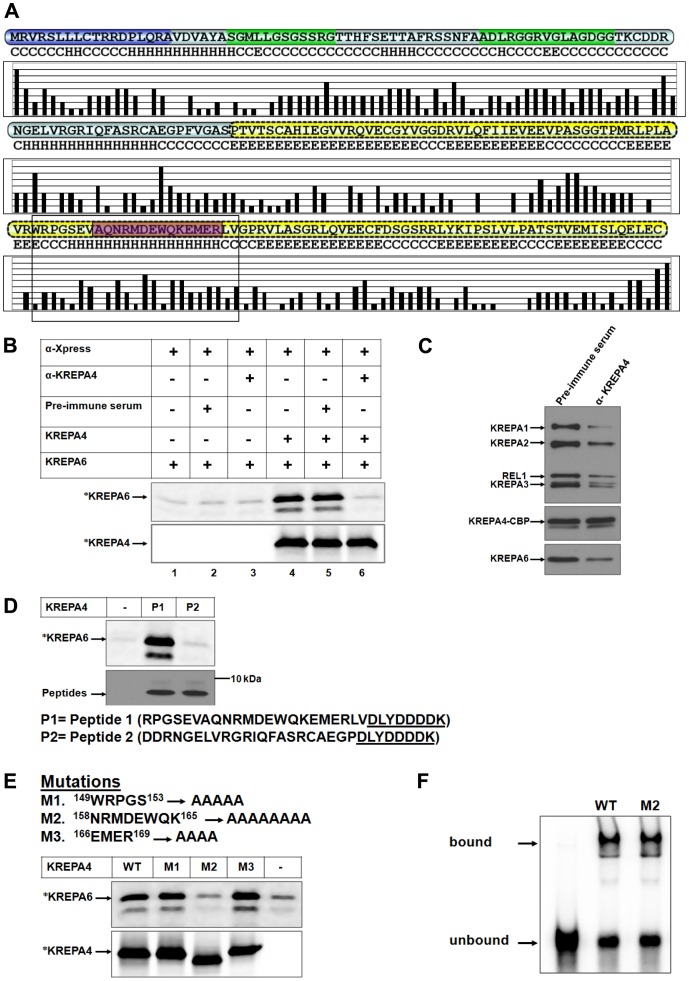
Figure 2. An antibody against the α-helix, or mutagenesis of this region inhibits the interaction with KREPA6.
(A) Solvent accessibility prediction of residues in KREPA4 secondary structure. Higher bars indicate hydrophilic exposed residues while lower bars indicate hydrophobic buried residues. The α-helix of KREPA4 OB-fold, hypothesized to mediate interaction with KREPA6 is highlighted by a box. The peptide sequence used for generating anti-KREPA4 antibody is highlighted in purple. (B, upper panel) CoIP of 35S-methionine labelled (*) KREPA6 with unlabelled full length KREPA4, using antibody against the KREPA4 Xpress tag (α -Xpress). Table indicates the presence or absence of the α-KREPA4 antibody and the pre-immune serum, as well as whether proteins are present singly or mixed together. (B, lower panel) The amount of 35S-methionine labelled (*) KREPA4 present in the CoIP is shown. When both proteins were present together, only one was radiolabelled at a time, as indicated. (C) The TEV eluate from KREPA4 TAP-tagged cells was treated with anti-KREPA4 antibody (α-KREPA4) or pre-immune serum as indicated, subjected to calmodulin affinity purification and eluates analysed by western blotting with antibodies as indicated. (D, upper panel) CoIP of 35S-methionine labelled (*) KREPA6 in the presence of KREPA4 peptide fragments as indicated. Lane marked with (−) shows the level of KREPA6 retained by non-specific binding to the beads, in the absence of KREPA4 peptides. (D, lower panel) The amounts of KREPA4 peptides present in the CoIP, as determined by western analysis with anti-Xpress antibody. The sequences of both peptides are indicated. Presence of the Xpress tag (underlined) on both peptides enabled the use of anti-Xpress antibody for the CoIP. (E) Summary of the KREPA4 α-helix mutations: the mutated residue positions are indicated. Upper panel CoIP of 35S-methionine labelled (*) KREPA6 in the presence of unlabeled wild-type (WT) or mutant (M) full length KREPA4 using antibody against the KREPA4 Xpress tag. Lane marked with (−) shows the level of KREPA6 retained by non-specific binding to the beads, in the absence of KREPA4. Lower panel Equimolar amounts of 35S-methionine labelled (*), wild-type or mutated KREPA4 proteins present in the CoIP are shown. When both proteins were present together, only one was radiolabelled at a time, as indicated. In case of M2, the alanine substitution has changed the overall charge and therefore, the electrophoretic migration of this mutant in comparison to the wild-type KREPA4. (F) Gel shift assay to monitor the gRNA-binding activity of equal amounts of wild-type (WT) and mutant (M2) KREPA4 proteins. The bound and free radiolabelled gA6 [14] gRNA is indicated.