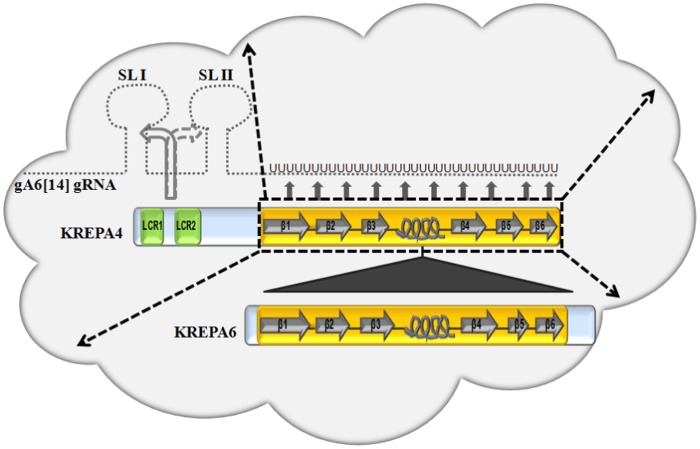
Figure 7. Proposed model for the RNA and protein interactions of KREPA4 in the 20S editosomes.
The editosome complex is depicted in the form of a cloud. Full length KREPA4 and KREPA6 proteins are shown as a part of the complex. The secondary structure of their OB-folds is represented schematically. Based on the current in vitro studies, the α-helix of the KREPA4 OB-fold is shown to interact directly with KREPA6 (filled triangle), while the entire OB-fold is shown to form stable associations with the editosome complex (thick, dashed arrows). In addition, based on previous in vitro RNA binding studies, the OB-fold of KREPA4 also mediates a high-affinity binding to the gA6 [14] gRNA oligo(U) tail (filled arrows), and this contact is stabilized by the LCRs through sequence-specific interactions with the guide stem–loop elements (curved arrows) [46]. The interaction of KREPA4 with stem–loop I (SL I) is preferred over that with stem–loop II (SL II) and is indicated by a solid curved arrow versus a dashed curved arrow, respectively. The oligo (U) tail of the gRNA is extended to fit the model.