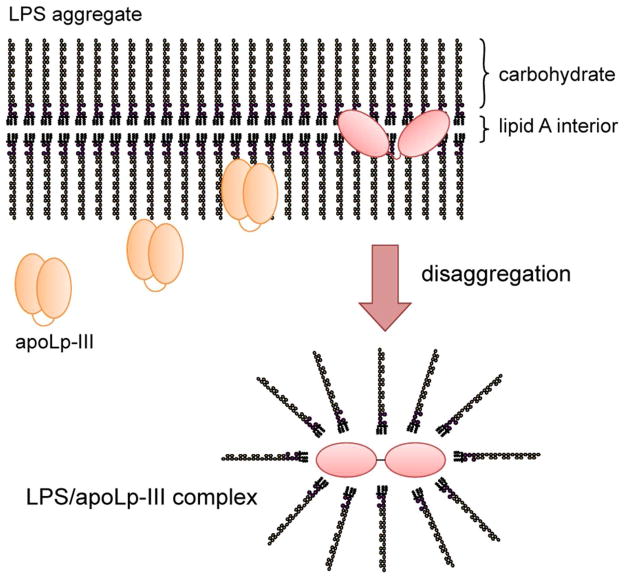
Figure 7.
Proposed model of the binding of apoLp-III to LPS. ApoLp-III first associates superficially to the LPS carbohydrates which protrude into the aqueous environment. Penetration into the interior of the LPS micelles provide access to the hydrophobic Lipid A region, which is then followed by LPS disaggregation. A conformational change of the protein allows direct interaction of the hydrophobic protein interior with the lipid A region, leading to the formation of a stable apoLp-III/LPS complex. The resulting product is an assembly of 4 apoLp-III and 24 LPS molecules with a size of ~ 400 kDa.