Figure 1.
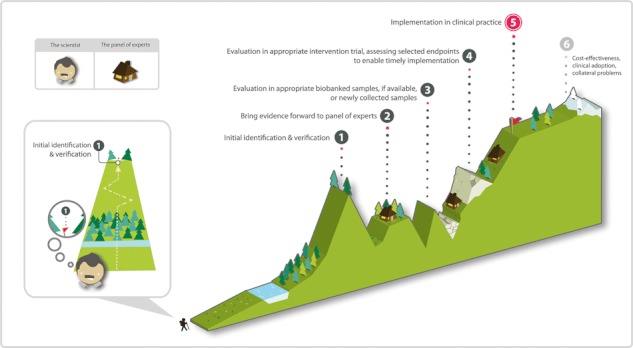
Implementation of novel biomarkers represents a substantially harder challenge than initially thought by scientists. As shown on the left-hand side of the cartoon, the current belief is that the major efforts are required during initial identification and verification of proteomics biomarkers. Implementation in the clinic is conceived as being simply a matter of continuing uphill, the ‘last few steps’ to the red flag. However, we argue that this is not true. While initial identification, verification and establishment of an appropriate analytical platform are without doubt major steps, even more substantial efforts are required on the road to actual implementation, which evidently is much longer than anticipated, and full of risks and additional obstacles. Among the major challenges are access to specific knowledge, sufficient funding, access to appropriate specimens, demonstration of reproducibility and performance of interventional trials. We propose support by a multidisciplinary panel immediately after initial verification, to accompany scientists on this road to implementation and to help avoid potentially useful biomarkers failing to reach the clinic. Once implementation in the clinic has been accomplished, the process does not stop, as cost-effectiveness, clinical adoption and collateral problems still must be monitored.
