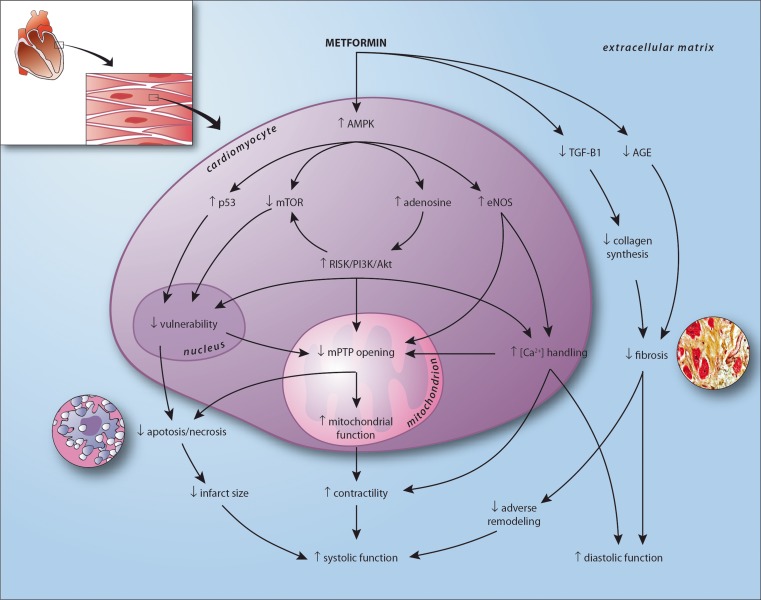
Fig. 2.
visualization of the proposed cardioprotective mechanism of action of metformin in the human heart after myocardial infarction, resulting in improved systolic and diastolic function. In experimental models metformin has been consistently associated with enhanced phosphorylation of AMP activated protein kinase (AMPK) [12–24]. In the myocardium, characterized by high energy demands and low energy reserves, AMPK plays a pivotal role in maintaining metabolic homeostasis [18–21]. Metformin-induced AMPK phosphorylation may be mediated by inhibition of complex 1 of the respiratory chain, by upstream activation of the tumor suppressor gene liver kinase B1 (LKB1), or by decreased AMP–deaminase activity [21–24]. AMPK phosphorylation leads to activation of the Reperfusion Injury Salvage Kinase (RISK) pathway including phospatidylinositol-3-kinase (PI3K) and Akt pathways [17, 26], upregulation of the tumor suppressor gene p53 [27], inhibition of mammalian target of rapamycin (mTOR) [19], and upregulation of endothelial nitric oxide synthase (eNOS) [1, 13]. Activation of the RISK pathway and eNOS improves mitochondrial function and inhibits opening of the mitochondrial permeability transition pore (mPTP) [26]. The mPTP is a major mediator of myocardial reperfusion injury. Opening of the mPTP results in ATP depletion and cell death [25, 26]. Further, prevention of mPTP opening stimulates mitochondrial respiration, improving ATP availability and cellular function [25]. Upregulated p53 and inhibited mTOR, partly RISK pathway mediated, are associated with decreased cellular vulnerability by preventing post-mitotic cell death and improved resilience to ischemia related injury [19, 27]. Metformin mediated eNOS production, next to increasing nitric oxide production, enhances sodium pump activity causing decreased intracellular calcium levels [1]. In infarcted tissue, this may attenuate microvascular obstruction and thereby prevent mPTP mediated cell death [28]. In functional myocardium, optimized calcium handling results in improved contractility and relaxation [1]. Further, independent of AMPK, metformin inhibits transforming growth factor (TGF)-β1 myocardial expression, decreasing collagen synthesis and preventing fibrosis [29]. Metformin may also attenuate cardiac fibrosis by directly inhibiting advanced glycation endproduct (AGE) formation [30]. Also, metformin is associated with a decrease in dipeptidyl peptidase-4 activity and an increase in circulating levels of glucagon-like peptide 1 [31]. In a porcine model of ischemia and reperfusion injury, stimulation with a analogue (exenatide) resulted in a reduction of infarct size [32]. Another target of metformin may be the increase of glucose utilisation of the heart. The adult heart mainly relies on fatty acids utilisation, and switches back to glucose when damaged. However, metabolic flexibility of the failing heart is limited, and facilitation of glucose utilisation by metformin via increase of glucose transporters (GLUT-1 and GLUT-4) may explain its salutary effects on the cardiac function [12, 33]