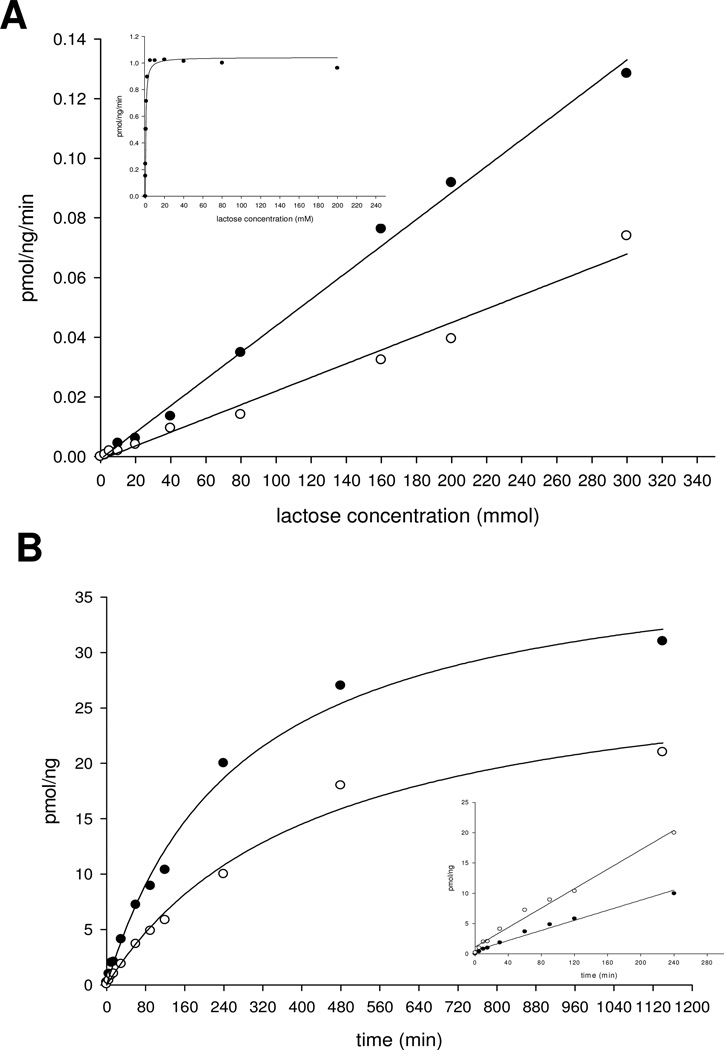
Figure 4.
(A) The catalytic activities of the α3Gal-T mutants 280SGG282 (○) and 280AGG282 (●) with UDP-GalNAc as the donor substrate at different concentrations of lactose. The insert shows the catalytic activity of the wild-type α3Gal-T with UDP-Gal as the sugar donor at different concentrations of lactose. Each assay was carried out for 15 min with 1 µg of enzyme, as described in Materials and Methods. (B) Time course of GalNAc transfer with 1 µg of mutant enzymes 280SGG282 (○) and 280AGG282 (●) at 200 mmol lactose and 500 µmol UDP-GalNAc.