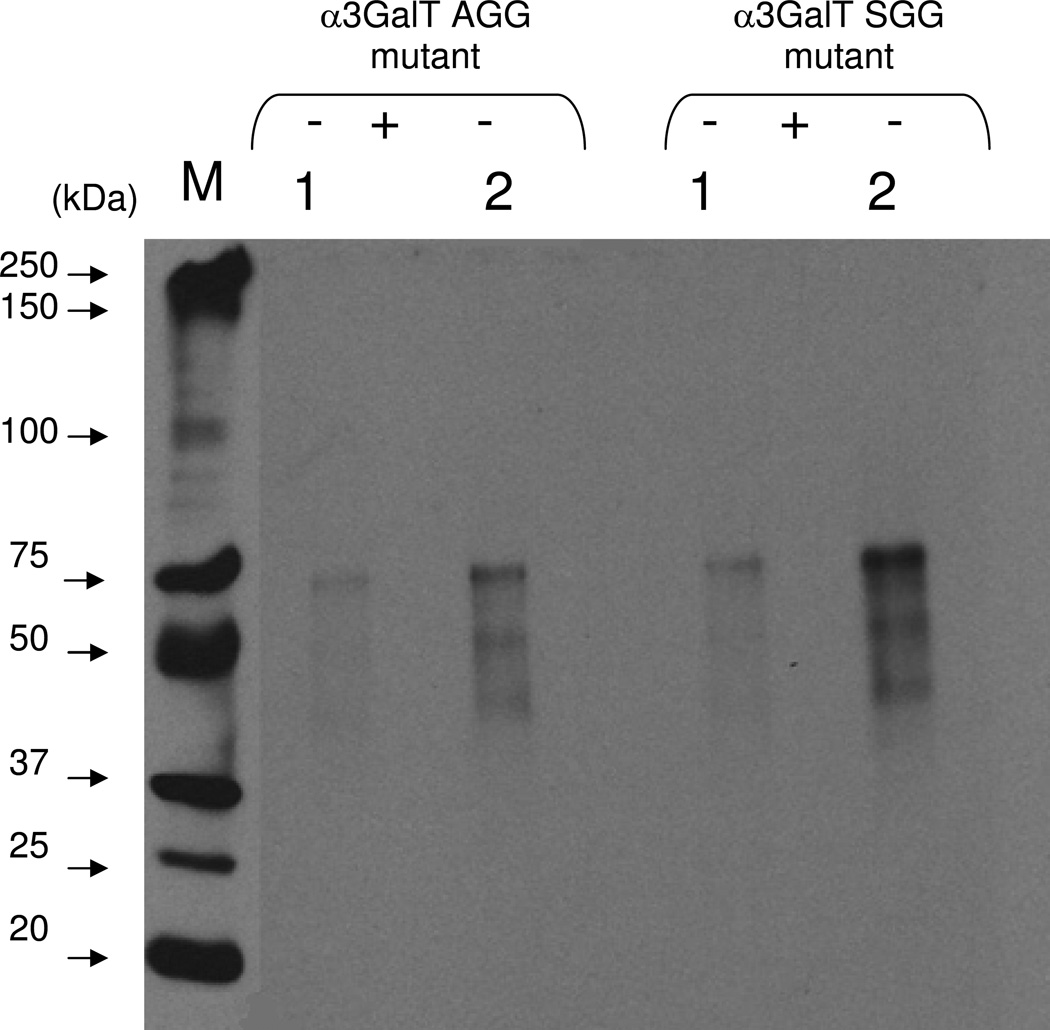
Figure 8.
Chemoenzymatic detection of the transferred 2-keto-Gal on asialofetuin. The transfer of 2-keto-galctose to LacNAc residues on the N-glycans chains of asialofetuin by the α3Gal-T mutant enzyme SGG and AGG was monitored by linking with the AOB, followed by Western blotting and chemiluminescence detection. The chemiluminescence was detected only in the samples that contained UDP-2-keto-Gal and mutant enzyme and 25 ng (1) and 50 ng (2) of asialofetuin. After the transfer of 2-keto-Gal, asialofetuin samples were treated with PNGase F (see Materials and Methods section), which removes the N-glycan chains from the protein (+). In contrast to the untreated samples (−), the PNGase F-treated samples (+) exhibited no chemiluminescence indicating that the transfer of 2-keto-Gal is selective for the glycan portion of asialofetuin.