Figure 1.
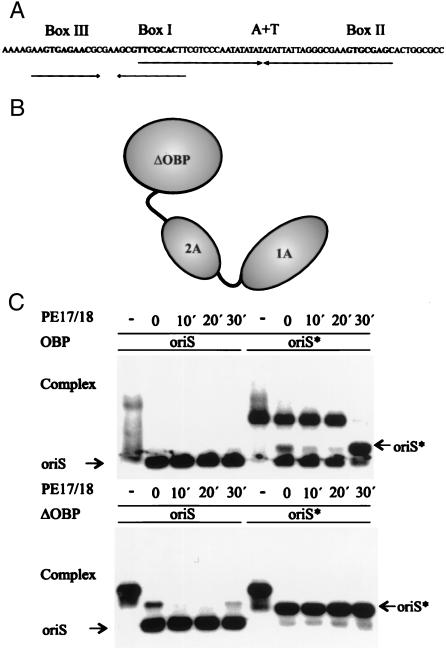
The N-terminal helicase domains are required for the formation of a stable complex between OBP and oriS*. (A) Schematic representation of HSV-1 oriS. Boxes I, II, and III represent recognition sequences for OBP in oriS. The position of the AT-rich spacer sequence is also shown. The arrows represent the two palindromes in oriS. (B) Schematic representation of OBP. The helicase domains are designated 1A and 2A. ΔOBP is the C-terminal DNA binding domain. Note that OBP is depicted as a monomer but exists as a dimer in solution. (C) Autoradiographs of gel retardation experiments with OBP, oriS, and oriS* (Upper) and HisΔOBP, oriS, and oriS* (Lower). OriS* was produced from duplex oriS by heat treatment as described in Materials and Methods. A 1,000-fold excess of a box I duplex oligonucleotide (PE17/18) was added as a competitor to preincubated OBP-DNA complexes for the times indicated.