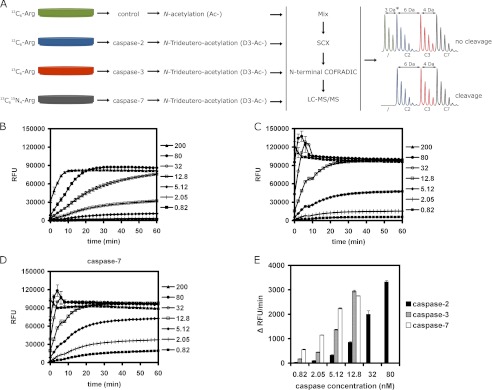
FIGURE 1.
COFRADIC setup for identifying human caspase-2 substrates and the activity of recombinant caspases used in the study. A, SILAC was used to label four differently treated A549 cell lysates. One part of the 12C6-labeled lysate served as control and another part was treated with caspase-2, whereas 13C6- and 13C615N4-labeled lysates were treated with recombinant human caspase-3 and -7, respectively. A differential acetylation step was introduced to distinguish between the control and the caspase-2-treated sample. N-Hydroxysuccinimide trideutero-acetate (D3-Ac-) was used in all caspase-treated samples and non-deuterated N-hydroxysuccinimide acetate (Ac-) in the control sample. After caspase treatment, equal amounts of samples were mixed, subjected to strong cation exchange chromatography (SCX), and N-terminal peptides were isolated by N-terminal COFRADIC and then analyzed by LC-MS/MS. Note that the actual mass difference between the control and caspase-2-treated samples (*) depends on the number of lysine residues in the peptide. Spectra are shown for a peptide without lysine residues and a Nα-(trideutero-)acetyl-group. Every additional lysine residue carried one additional (trideutero-)acetyl-group leading to an extra mass difference of 3-Da per lysine. B–D, in vitro caspase activity was measured for 60 min at 37 °C using 50 μm synthetic peptide substrates: VDVAD-AMC for caspase-2 (B) and DEVD-AMC for caspase-3 (C) and -7 (D) using the indicated caspase concentrations (nm). At 200 nm, all three caspases show maximal activation within minutes. E, results of the caspase activity assay show the initial velocity (change in fluorescence over time: Δ RFU/min) for reactions with a linear initial phase during the first 20 min. Caspase-7 was the most, and caspase-2 the least active protease.