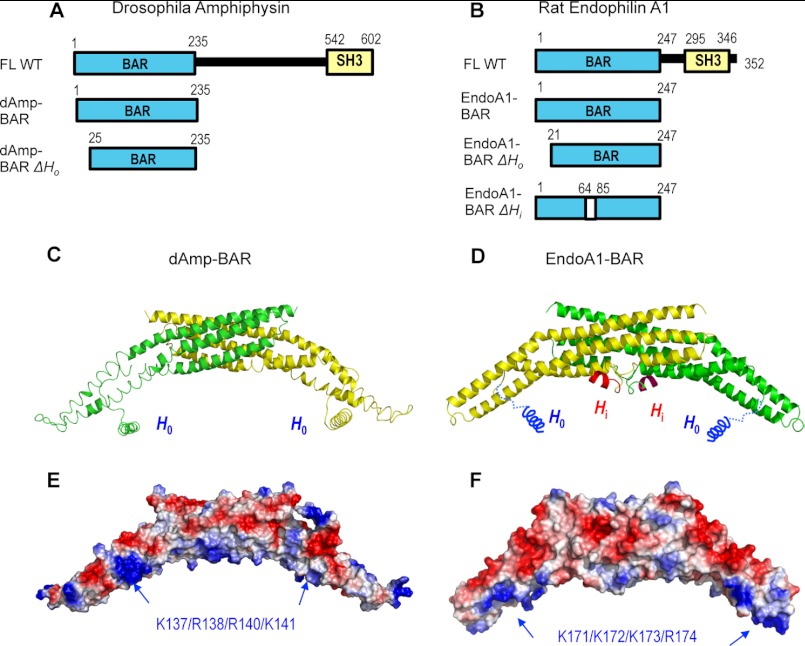
FIGURE 1.
Structures of dAmp-BAR and EndoA1-BAR. A, diagrams of Drosophila amphiphysin constructs (FL, full-length; SH3, Src homology 3). B, diagrams of rat endophilin A1 constructs. ΔHi lacks residues 65–84. C, the crystal structure of dAmp-BAR (Protein Data Bank code 1URU) (15) showed that this domain forms a crescent-shaped dimer of two coiled coils (green and yellow), each made of three long kinked α-helices. The helical structure of H0 for dAmp-BAR was modeled. D, rat EndoA1-BAR has a similar molecular shape and charge distribution (Protein Data Bank code 2C08) (24). The main difference is that it has Hi in the middle of the concave face (only part of the Hi structure is structurally defined). E and F, electrostatic potential calculation shows that the concave faces of dAmp-BAR and EndoA1-BAR have cationic patches that would interact with anionic membranes. Locations of mutated cationic residues are indicated by blue arrows. Red and blue colors in surface representations qualitatively indicate negative and positive electrostatic potentials, respectively.