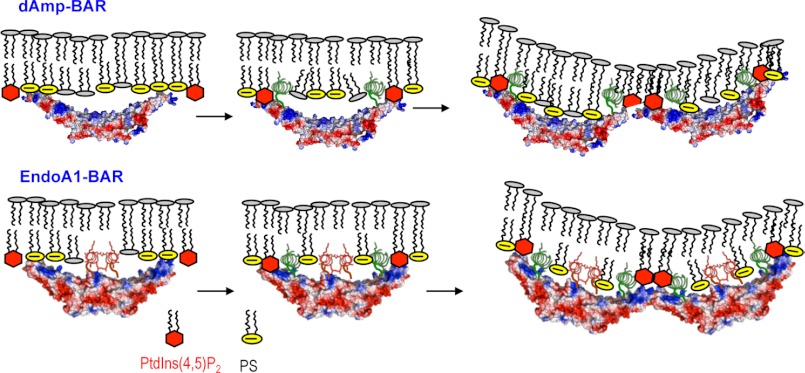
FIGURE 7.
Proposed mechanisms of membrane deformation by dAmp-BAR and EndoA1-BAR. The initial plasma membrane binding of dAmp-BAR is driven by a nonspecific electrostatic interaction between cationic patches of the concave surface and the anionic membrane surface containing PS (yellow circles) and PtdIns(4,5)P2 (red hexagons). PtdIns(4,5)P2 is more effective than PS at inducing partial membrane penetration of H0 (green spirals) because PtdIns(4,5)P2 binding induces the formation of H0 that forms part of the PtdIns(4,5)P2 binding site as is the case with H0 of epsin1 ENTH (14). PtdIns(4,5)P2-depedent membrane penetration of H0 not only enhances the membrane affinity of dAmp-BAR but also is essential for its self-association, which is also important for its membrane deformation. For EndoA1-BAR, initial membrane binding is driven by both a nonspecific electrostatic interaction between cationic patches of the concave surface and the anionic membrane surface and PtdIns(4,5)P2-indepedent membrane penetration of Hi (red spirals). Thus, EndoA1-BAR has higher intrinsic membrane affinity and membrane penetration power than dAmp-BAR. As is the case with dAmp-BAR, PtdIns(4,5)P2 binding then induces the formation and the partial membrane penetration of H0, which leads to enhanced membrane affinity and protein self-association. The BAR domains are shown in surface representation with red and blue indicating negative and positive electrostatic potentials in qualitative terms, respectively. Only 4-mers are shown for illustration, but both N-BAR domains form much larger aggregates.