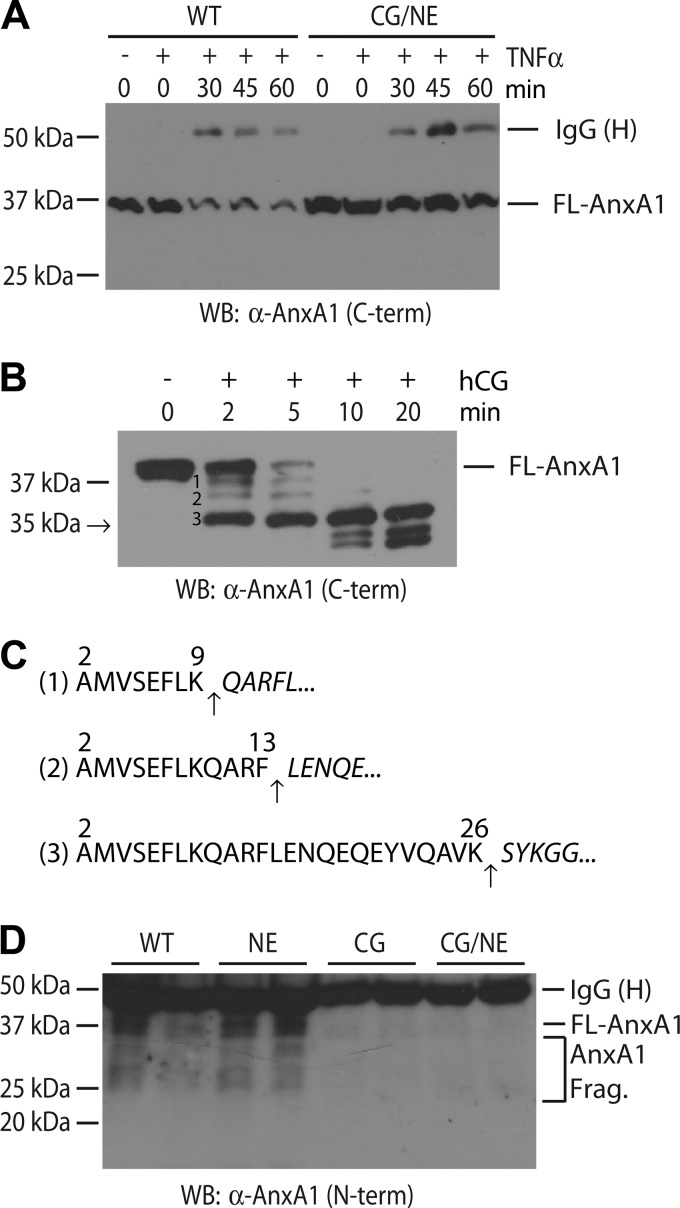
FIGURE 2.
CG modulates the secretion and cleavage of AnxA1 by mouse neutrophils. A, loss of cell-associated AnxA1 during neutrophil activation. TNF-α-primed, IC-stimulated WT and CG/NE neutrophils were harvested at the indicated time points and cell-associated full-length (FL) AnxA1 was analyzed by Western blot (WB) using a C terminus directed rabbit antibody. The heavy chain (H) of rabbit anti-OVA IgG, used to generate plate-bound IC, was also solubilized during the neutrophil harvesting process. B, human CG (hCG) rapidly cleaved recombinant murine AnxA1 to release N-terminal peptides. C, N-terminal sequencing revealed the cleavage sites (arrows) that generated the indicated products in B. The numbering system refers to amino acid position in full-length AnxA1. D, CG was required for the efficient release of AnxA1 in vivo. Supernatants obtained from reverse passive Arthus reaction in six-day-old subcutaneous air pouches were acetone-precipitated, and total proteins were probed for full-length AnxA1 and AnxA1 fragments (AnxA1 Frag.) by Western blot using an N terminus-directed goat antibody.