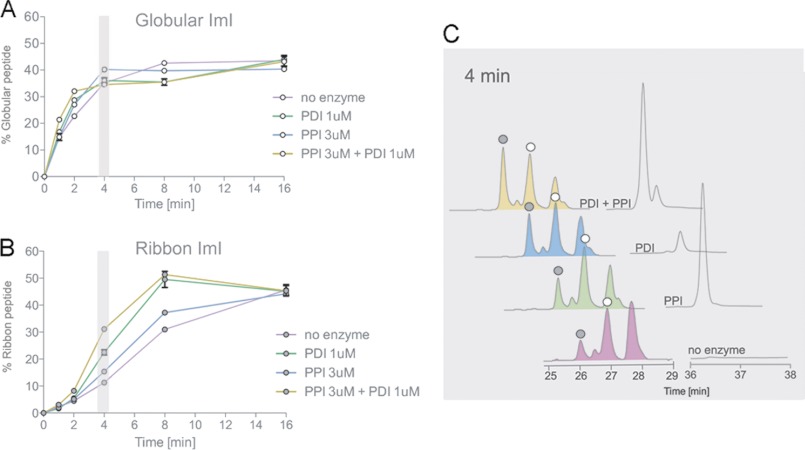
FIGURE 5.
Analysis of PDI- and PPI-assisted folding of α-ImI. Oxidative folding studies were performed in the presence of 0.1 mm GSH, 0.1 mm GSSG, and 20 μm linear peptide with and without Conus PDI and/or PPI B. Folding reactions were acid-quenched at 0, 2, 4, 8, 16, and 32 min and analyzed by reversed-phase chromatography. Relative abundances of the fully folded globular (A) and fully folded ribbon peptide (B) were determined by reversed-phase chromatography as shown in C and plotted against time points of folding. Plotted values are averages from three independent experiments (mean ± S.D. (error bars)). C, reversed-phase chromatograms of folding reactions quenched after 4 min. White circles denote the folded globular peptide, and gray circles show the ribbon isomer, as determined by their characteristic elution profiles (28) and co-elution experiments.