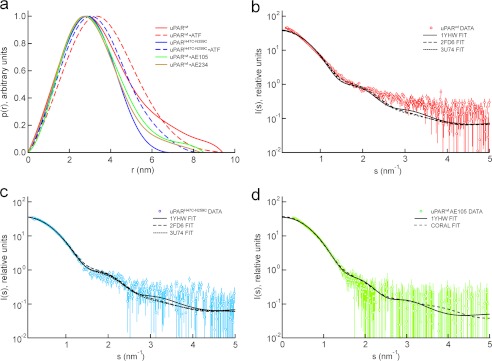
FIGURE 1.
Comparison between SAXS data and crystal structures. The pairwise interatomic distance distributions were determined from the scattering profiles for uPAR in six different states and are displayed as a p(r) function in a. The p(r) functions clearly show that uPARwt is the most extended species and that the disulfide-constrained uPARH47C/N259C represents the most compact species. The complexes with two different 9–10-mer peptide antagonists (AE105 and AE234) display an intermediate compactness. The best fits of the known crystal structures to the SAXS data are shown for uPARwt (b), for uPARH47C/N259C (c), and for uPARwt·AE105 complexes (d). Both uPARH47C/N259C and uPARwt·AE105 complexes fit the corresponding crystal structures solved for these states, whereas the unoccupied uPARwt is not adequately described by any of the known structures.