FIGURE 5.
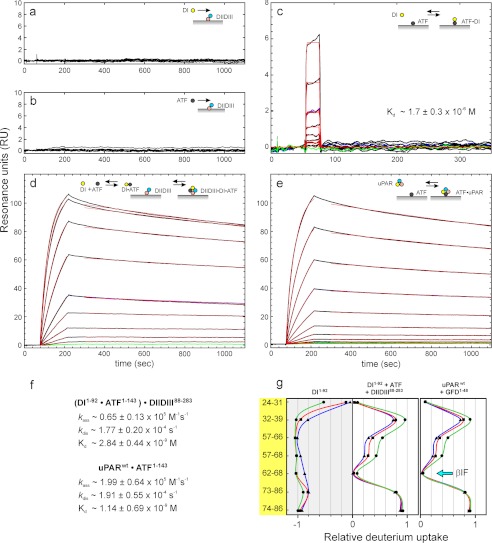
Ligand binding kinetics for intact uPAR and its fragments DI and DIIDIII using surface plasmon resonance. To probe the uPA binding potential of unoccupied uPAR with a detached DI, we assessed the binding kinetics of DI(1–92) and ATF(1–143) to immobilized DIIDIII(88–283) either alone or in combination. a and b show that neither DI nor ATF binds as a single component to immobilized DIIDIII (218 resonance units (RU), ∼9 fmol/mm2) when tested up to 2 μm. c shows that DI(1–92) binds weakly to immobilized ATF with an equilibrium dissociation constant (Kd) of 1.7 μm, which is similar to the one we measure for ATF(1–143) binding to immobilized DI (Kd of 1.6 μm; data not shown). In contrast, the sensorgrams in d reveal a tight binding to immobilized DIIDIII, when a serial 2-fold dilution of DI(1–92) (8 nm to 2 μm) is tested in the presence of 1 μm ATF(1–143). The second order rate constants for the interaction of ATF·DI complexes with immobilized DIIDIII were calculated assuming a Kd of 1.7 μm for the ATF·DI interaction in solution (c). For comparison, the interaction between a serial 2-fold dilution of intact uPAR (0.6–200 nm) and immobilized ATF is shown in e. Repeat analyses are shown as blue curves, and buffer runs are shown as green curves in c–e. Fits to dissociation and association phases are shown as thin red lines, and the derived kinetic rate constants are shown in f. g, the solvent accessibility of DI(1–92) was measured by HDX-MS either as an isolated component or in a trimolecular complex, DI·ATF·DIIDIII, which was preassembled by incubating a mixture of the individual component (30, 60, and 60 μm, respectively) for 60 min before exchange was initiated for 10, 100, and 1000 s using same color coding as in Fig. 4.