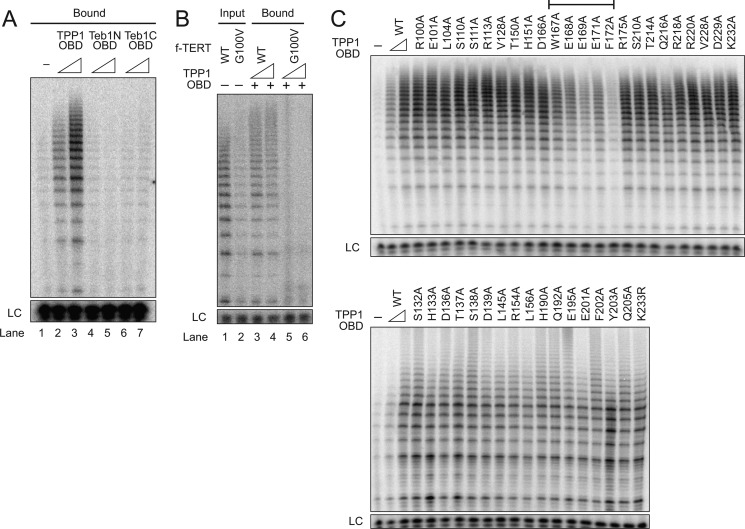
FIGURE 4.
Identification of a TPP1 OBD surface required for holoenzyme interaction. A, extract from cells co-expressing hTR and f-TERT was used for holoenzyme binding to Ni-NTA-agarose with His6-tagged TPP1 OBD or the His6-tagged N-terminal or C-terminal OBD of Tetrahymena Teb1. Either 100 pmol or 1 nmol of each purified OBD was prebound to the resin. A negative control monitored holoenzyme binding to resin lacking prebound OBD (lane 1). B, extract from cells co-expressing hTR and either WT or G100V f-tagged TERT was used for holoenzyme purification. The holoenzymes were then allowed to interact with the TPP1 OBD immobilized on Ni-NTA-agarose. Either 20 or 200 pmol of TPP1 OBD was prebound to the resin, and activity was assayed directly on resin without elution (lanes 3–6). Activity assays of the input purified holoenzymes (lanes 1 and 2) used 10% of the amount allowed to bind to TPP1 OBD. C, telomerase holoenzyme binding to resin-immobilized WT TPP1 OBD or the TPP1 OBD variant indicated. Each panel begins with a negative control monitoring holoenzyme binding to resin lacking a TPP1 OBD. Either 20 or 200 pmol of WT or 200 pmol of sequence variant TPP1 OBD was prebound to the resin. Activity was assayed directly on resin without elution. The bracket indicates variants with reproducibly and severely reduced co-immunopurification of holoenzyme activity. LC, loading control.