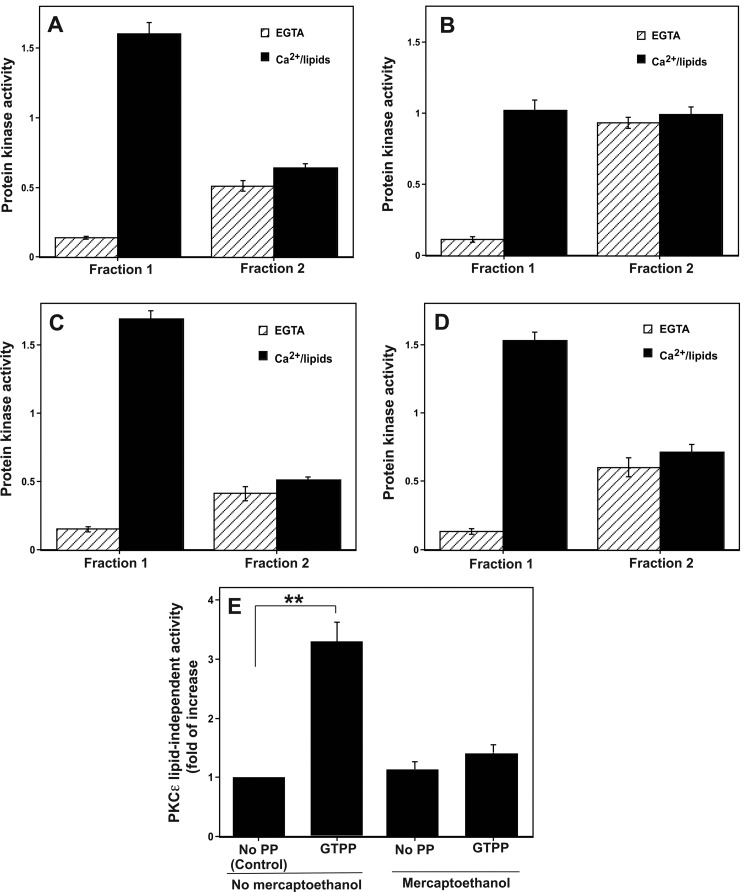
FIGURE 5.
Generation of constitutively active form of pan-PKC and PKCϵ in cells treated with GTPP. A, shown is elution of pan-PKC activity from control cells into two fractions. PC12 cells were homogenized in a buffer without thiol agents. A detergent-soluble cell extract (cytosol and membrane) was prepared and applied to a small (0.5 ml) DEAE-cellulose (Whatman DE52) column. The bound PKC isoenzymes (lipid-dependent forms) were eluted with 1.25 ml of 0.1 m NaCl (Fraction 1), whereas the protein kinase activity exhibiting less dependence on cofactors was eluted with 1.25 ml of 0.25 m NaCl (Fraction 2). PKC activity was measured with neurogranin peptide as a substrate. B, shown is elevation of the constitutively active form of PKC in cells treated with GTPP. PC12 cells were treated with GTPP (0.2 μg/ml) for 30 min, cell extracts were prepared, and the fractions 1 and 2 were isolated as above. C, shown is reversal of GTPP-induced PKC modification by mercaptoethanol. GTPP-treated PC12 cells were homogenized in a buffer with 10 mm 2-mercaptoethanol, and fractions 1 and 2 were isolated as described above. D, shown is prevention of GTPP-induced PKC modification by catalase. PC12 cells were initially treated with PEG-catalase for 18 h, then treated with GTPP, and fractions 1 and 2 were isolated. E, shown is PKCϵ activity in control and GTPP-treated cells. PC12 cells were treated with GTPP, and then PKCϵ activity was measured by an immune complex kinase assay using anti-PKCϵ rabbit polyclonal antibodies. PKCϵ activity was determined with and without lipids in the presence and absence of 2-mercaptoethanol (5 mm) as described under “Experimental Procedures.” The values represent the means and S.E. of three estimations. Statistical difference between means is indicated with asterisks (**, p < 0.001). PP, PP, polyphenol.