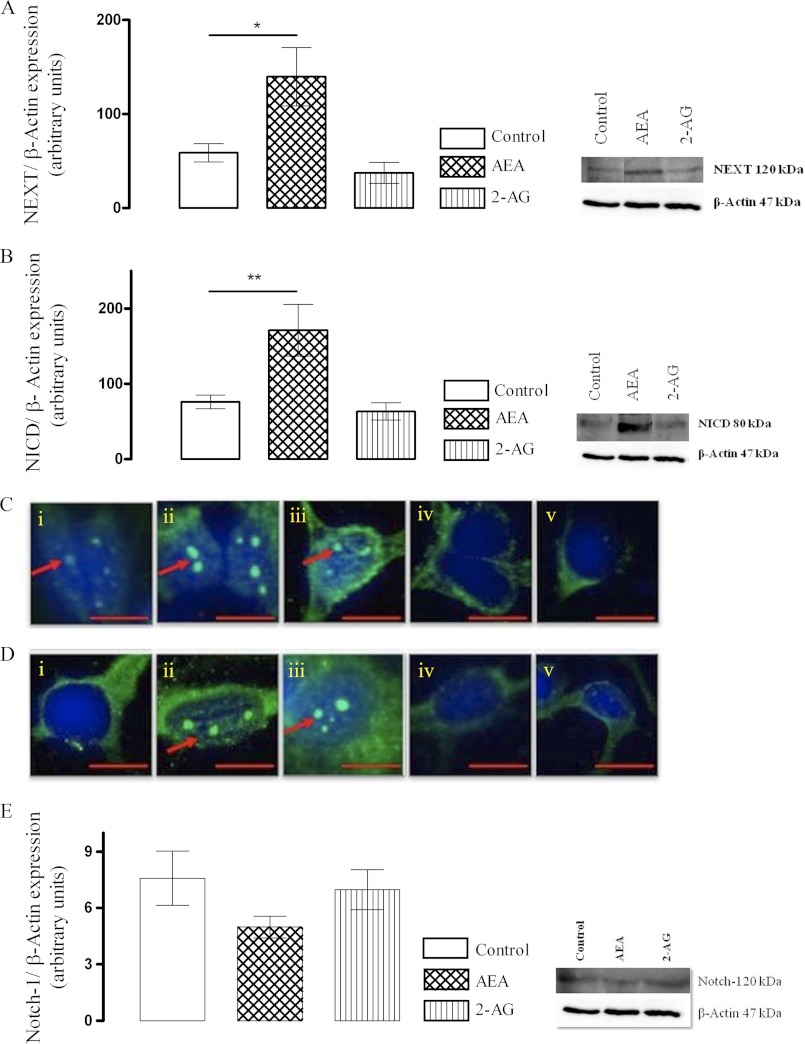
FIGURE 1.
AEA up-regulates Notch-1 receptor cleavage to enhance cellular NEXT and NICD. A, cortical neurons were exposed to AEA (10 nm) or 2-AG (10 nm) for 24 h, and cellular NEXT was assessed by Western immunoblot. AEA enhanced site 2 cleavage of full-length Notch-1 receptor and enhanced generation of NEXT (*, p < 0.05 versus control, ANOVA, n = 6). B, AEA (10 nm) augmented subsequent site 3 cleavage by γ-secretase and generated NICD as measured by Western immunoblot (**, p < 0.01 versus control, ANOVA, n = 6). C, cortical neurons were exposed to AEA (10 nm), URB 597 (1 μm), 2-AG (10 nm), or URB 602 (100 μm) for 6 h. Nuclear translocation of NICD was assessed as a measure of Notch-1 receptor cleavage by immunocytochemistry. Exposure of neurons to AEA or URB 597 up-regulated NICD generation and its nuclear translocation. Sample confocal images of NICD immunostaining of cells treated with control medium (i), AEA (ii), URB 597 (iii), 2-AG (iv), and URB 602 (v) for 6 h are shown. In control cells constitutive nuclear NICD was observed. D, the positive regulatory effects of AEA and URB 597 on Notch-1 receptor cleavage and its nuclear translocation in cortical neurons persisted at 24 h. Sample confocal images of NICD immunostaining of cells treated with control medium (i), AEA (ii), URB 597 (iii), 2-AG (iv), and URB 602 (v) for 24 h are shown. Arrows indicate nuclear NICD expression. Scale bar, 10 μm, n = 6. E, exposure to AEA (10 nm) reduced (not statistically significant, p = 0.07, n = 6) full-length Notch-1 receptor in neurons possibly as a result of its enhanced processing.