FIGURE 2.
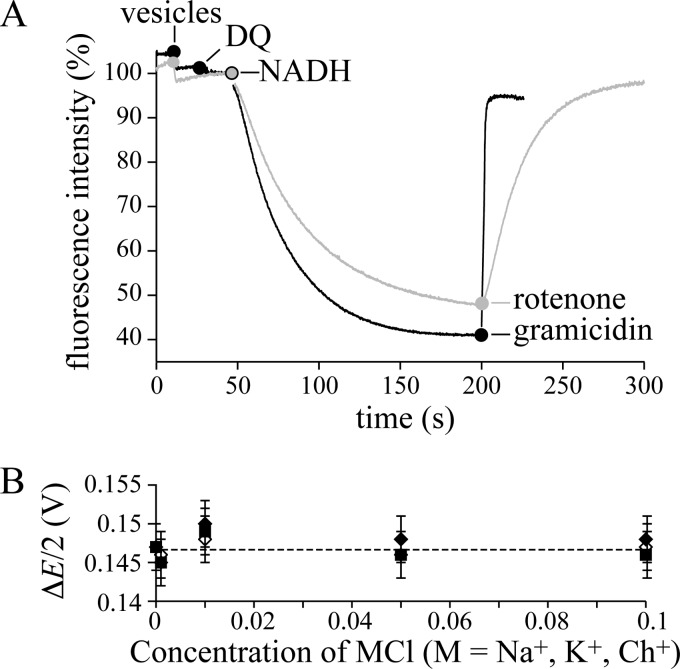
Mitochondrial complex I is a redox-coupled proton pump. A, quenching of the ACMA fluorescence demonstrates ΔpH formation in BtPLs and BtSMPs. Black: BtPLs (1.3 μg-protein ml−1), 200 μm DQ, and 200 μm NADH; 2 μg ml−1 gramicidin abolishes Δp. Conditions are: 10 mm Tris-SO4, pH 7.5, 125 mm sucrose, 25 mm KCl, 2 μg ml−1 valinomycin, 20 °C. Gray: BtSMPs (3.8 μg-protein ml−1), and 200 μm NADH; 200 nm rotenone stops proton translocation, and Δp dissipates. Conditions are: 10 mm Tris-SO4, pH 7.5, 125 mm sucrose, 80 mm KCl, 20 °C. B, the effect of Na+, K+ and Ch+ on the apparent Δp (ΔE/2), generated by ATP hydrolysis by SMPs. The rate of NADH:fumarate oxidoreduction was recorded as a function of ΔE, where ΔE (V) = −0.315 − RT/2F.ln{([NADH][fumarate])/([NAD+][succinate])} using 0.1 mm [NADH], 1 mm [NAD+], 0.5 mm [succinate], 0.025–40 mm [fumarate]). When the net rate is zero, ΔE/2 = Δp (assuming 4H+ per NADH). Conditions are: 10 mm Tris-SO4, pH 7.5, 32 °C, 0 to 100 mm MCl (M, Na+, K+, Ch+) with variable sucrose concentration (250 to 50 mm) to maintain the osmolarity. Error bars indicate S.D.