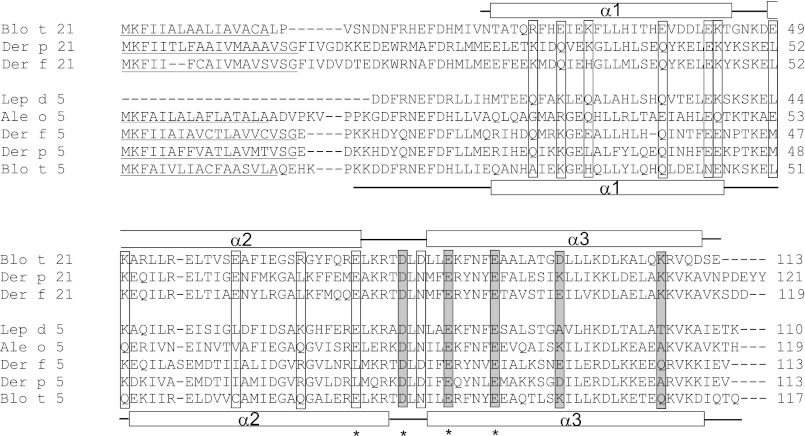
FIGURE 1.
Sequence alignment of Blo t 21 with group 21 and 5 allergens from other house dust mites (Der p 21, Der f 21, Blo t 5, Der p 5, and Der f 5) and less common storage product mites (Ale o 5 from Aleuroglyphus ovatus and Lep d 5 from Lepidoglyphus destructor). Seventeen charged residues (boxed) were chosen for site-directed mutagenesis. Between Blo t 21 and Blo t 5, 13 of the charged residues showed distinct properties, whereas four of the charged residues (marked by asterisks) were previously identified as IgE epitopes in Blo t 5, corresponding to residues Glu-74, Asp-79, Glu-84, and Glu-89 in Blo t 21. Mutation of the residues (gray shaded boxes) were found to cause a significant (>20%) drop in IgE binding in 6 of the 12 allergic patients. The signal peptide regions predicted by SIG-Pred are underlined. The boundaries of the secondary structures from the NMR structure of Blo t 21 and Blo t 5 are shown above and below the sequences, respectively. Note that the N-terminal 17 residues of Blo t 21 are highly unstructured and are not included in sample preparation and structure determination.