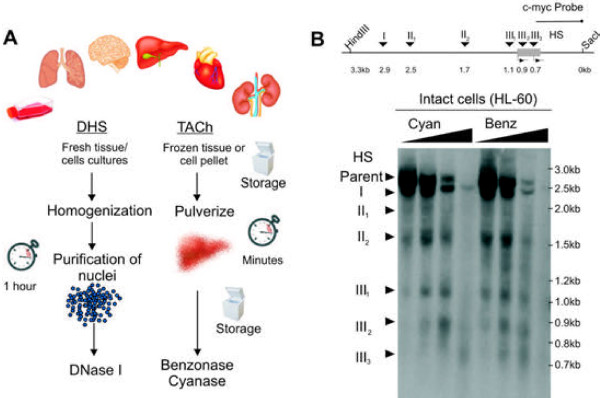
Figure 1.
Identification of nuclease hypersensitive regions in chromatin using TACh.(A) Side by side comparison of experimental setups for identification of nuclease hypersensitive sites using DNase I (DHS) versus Benzonase-Cyanase (TACh). (left panel) For DHS freshly isolated tissue is homogenized and nuclei are purified using protocols that take one to two hours. Purified nuclei are treated with various concentrations of DNase I. DNA fragments released by partial endonucleae digestion are isolated and can be analyzed by Southern blotting, qPCR or deep sequencing. (right panel) In contrast to the DHS procedure, TACh uses frozen tissue or cell pellets as starting material. Tissue is initially pulverized and divided into appropriate quantities, which can be stored frozen. Pulverized tissue is suspended in hypotonic nuclease buffer containing Benzonase or Cyanase at various concentrations and like for the DHS assay, DNA fragments released by partial endonuclease digestion can be analyzed by the same various methods. (B) Identification of nuclease accessible regions at the c-myc promoter in intact human HL60 cells using Benzonase or Cyanase. Detection of accessibility by Southern blotting and indirect end labeling is shown. (Top) Illustration of the human c-myc promoter with indication of probe annealing site and six previously published DNase I hypersensitive (HS) sites. (Bottom) Intact HL-60 cells, grown in suspension, were resuspended in hypertonic nuclease digestion buffer containing Benzonase or Cyanase. Digested DNA was purified and digested with HindIII and SacI, which releases a 3.3 kb fragment of the c-myc promoter. Partial nuclease digested fragments were detected using a biotinylated probe as indicated.