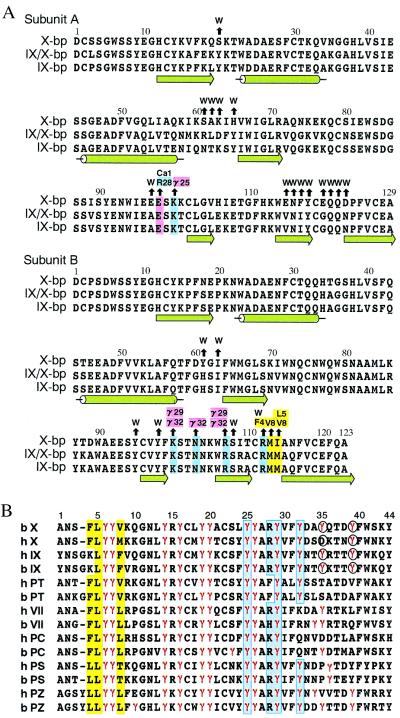
Figure 1.
Amino acid sequence alignments. (A) Anticoagulant proteins from snake venoms. Black arrows above the residues of X-bp point to amino acid residues of XGD1-44, Ca-1, or water molecules (W) with which they interact. Positively charged, negatively charged, and hydrophobic residues involved in those interactions are colored in blue, pink, and yellow, respectively. The numbering and secondary structure elements also are shown. (B) The Gla domains of vitamin K-dependent proteins (X, factor X; IX, factor IX; PT, prothrombin; VII, factor VII; PC, protein C; PS, protein S; PZ, protein Z) from human (h) and bovine (b). The numbering at the top refers to the bX sequence. Gla residues are denoted by γ and colored in red. Blue and yellow columns show hydrophilic and hydrophobic patches, respectively. Residues coordinating to the eighth Ca2+ ion are indicated in the circles.