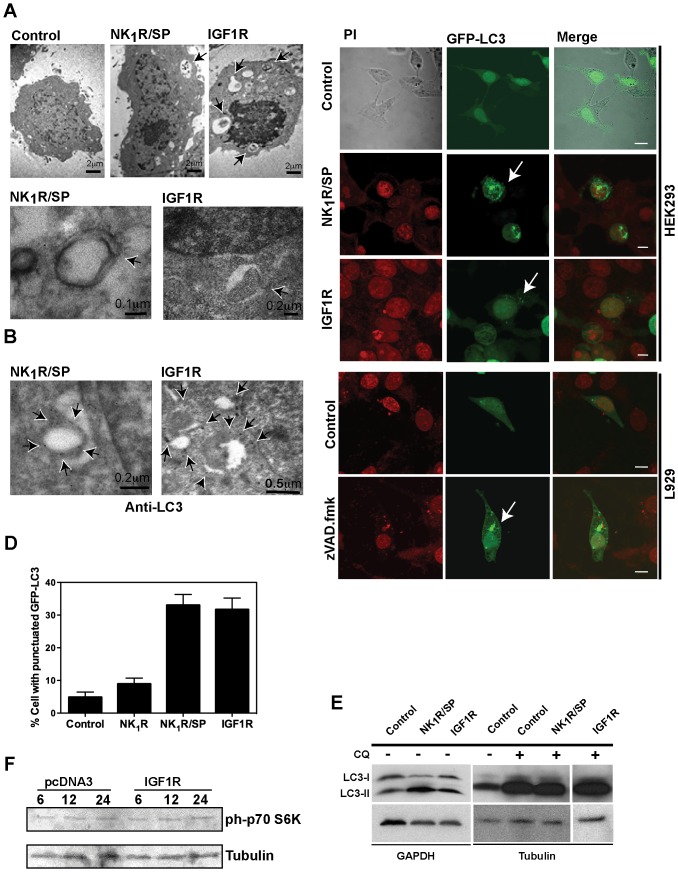
Figure 2. NR4A1-mediated vesicular cell death has autophagic features.
A, upper panels, presence of vesicles with cytoplasmic content (some marked by arrows) 12 h after cell death induction by either NK1R/SP or IGF1R, observed by electron microscopy. Bottom panels, examples of structures resembling autophagosomes about to close (arrows). B, Localization of endogenous LC3, examined by immunoelectron microscopy using antibody against LC3, 12 h after cell death induction by either NK1R/SP or IGF1R. Arrows indicate examples of LC3 associated with autophagosomes. For A and B, cells were transfected with empty vector (control), NK1R and exposed to SP, or IGF1R, as indicated. C, D, GFP-LC3 is redistributed during NR4A1-mediated vesicular cell death. HEK293 cells were co-transfected with GFP-LC3 and either the empty vector (control), NK1R and exposed to SP, or IGF1R. Examples of cells showing GFP-LC3 punctuated distribution are shown by confocal images in C. Percentage of cells with GFP-LC3 re-distribution, among GFP positive cells, are plotted in D. 4 fields per treatment were counted, in three independent experiments. Bars represent standard deviation. As a positive reference for autophagy, L929 cells were transfected with GFP-LC3 and exposed or not to caspase inhibitor zVAD.fmk. Nuclei were stained with Propidium Iodide (PI). Scale bar, 5 µm. E, LC3-II form accumulates during NR4A1-mediated vesicular cell death. HEK293 cells were transfected with the indicated expression vectors and total protein extracts were collected 24 hr after death induction to detect LC3 by Western blot. GAPDH or Tubulin were detected as loading reference. Autophagic flux did not seem to be impaired, since in the presence of Chloroquine (CQ) LC3-II accumulated furthermore, although there was not a detectable difference between CQ alone and CQ plus cell death inducers (even at lower exposure times). F, the autophagy inhibitor kinase mTOR is not activated during IGF1R-induced death. Protein extracts were collected at the indicated times (hr) after transfection with the empty vector pcDNA3 or IGF1R and the phosphorylation of the mTOR target p70 S6K was detected by Western blot. As a loading reference, tubulin was detected in the same blot. As can be observed, the basal level of phosphorylated p70 S6K did not change.