Abstract
Objective
Oxidative stress contributes to atherosclerosis, and evidence of enhanced oxidative stress exists in antiphospholipid syndrome (APS). In a non-lupus murine model, we evaluated whether anticardiolipin (aCL) antibodies could affect the oxidant/antioxidant balance as an early biochemical step of APS.
Methods
Hybridomas producing human and murine aCL and anti-β2-glycoprotein I (aβ2-GPI) monoclonal antibodies were injected into three groups of five female BALB/c severe combined immunodeficiency (SCID) mice. Corresponding hybridomas secreting non-antiphospholipid antibodies of the same isotype were employed as controls. Sera and organs were collected after 30 days. Paraoxonase (PON) activity, peroxynitrite, superoxide, nitric oxide (NO) and nitrotyrosine were measured in plasma. Expression of endothelial nitric oxide synthase and inducible nitric oxide synthase (iNOS) was assessed by western blot and immunohistochemistry.
Results
PON activity and NO (sum of nitrate and nitrite) levels were reduced in the human aCL IgG group (P<0.002 and P<0.04, respectively), whilst peroxynitrite and superoxide and expression of total antioxidant capacity of plasma were increased (P<0.01). PON and NO were decreased in the murine aβ2-GPI IgG and IgM aCL groups (P<0.03 and P<0.05, respectively). Nitrotyrosine was elevated in the human aCL IgG group (P<0.03). Western blotting showed reduced iNOS expression in the hearts of the IgG aCL group, confirmed by immunostaining. PON inversely correlated with IgG aCL titres (P<0.001), superoxide (P<0.008) and peroxynitrite levels (P<0.0009). Peroxynitrite and total IgG aCL were independent predictors of PON (P<0.0009 and P<0.02, respectively). Superoxide was the only independent predictor of NO (P<0.008) and of nitrotyrosine (P<0.002).
Conclusion
aCL antibodies are associated with the decreased PON activity and reduced NO that may occur in the preclinical phase of APS.
Keywords: Antiphospholipid antibodies, Oxidative stress, Nitric oxide, Total antioxidant capacity, Paraoxonase
The antiphospholipid syndrome (APS) is characterized by venous and arterial thromboses and recurrent miscarriages in persistent carriers of antibodies against negatively charged phospholipids [1, 2] and plasma proteins including β2-glycoprotein I (β2-GPI) coagulation proteins and complement factors [3–6].
Nitric oxide (NO) is the main endothelium vasodilator [7], and interference with NO synthesis induces vascular dysfunction [8], particularly in the early phases of atherosclerosis [9]. Furthermore, oxidation of lipids causes oxidative stress that is associated with atherosclerosis [10], both features of APS [11]. Anticardiolipin (aCL) antibody titres positively correlated to plasma levels of F2-isoprostanes, sensitive markers of in vivo lipid peroxidation [12], indicating enhanced oxidative stress in APS [13], and to decreased urinary excretion of NO metabolites [14].
An emerging concept in atherogenesis relates to paraoxonase (PON). This enzyme is carried in plasma by high-density lipoprotein and its major function is to prevent oxidation of low-density lipoprotein [15]. In primary APS, PON activity is reduced and correlated inversely with aCL titres and directly with the total antioxidant capacity of plasma [16]. Decreased PON activity, with increased oxidative stress and reduction of NO, may be involved in the early phases of APS.
To further evaluate the association between aCL antibodies and oxidative stress in vivo, we have tested whether aCL antibodies may affect the oxidant/antioxidant balance in an experimental non-lupus murine model.
Materials and methods
Hybridoma cells
The following murine hybridoma cell lines were injected into the mouse peritoneum: (i) hybridoma generating IS4, a human immunoglobulin (IgG) monoclonal antibody that binds to cardiolipin and β2-GPI, derived from patients with APS [17]; (ii) TW, a hybridoma cell line secreting human IgG that tested negative against cardiolipin and β2-GPI. This was used as a human IgG control (a kind gift of Thomas Winkler, Erlangen, Germany); (iii) hybridoma 2A1-A17.3, producing IgG1 anti-β2-GPI (aβ2-GPI); (iv) 16A3-14.11, producing IgM aCL [18]; (v) 29J3-119 and (vi) 16B4-2, producing IgG1 and IgM antibodies, respectively, which do not bind to β2-GPI or cardiolipin. These were used as negative controls as well as CBF7, the non-secreting mouse–human heteromyeloma fusion partner cell.
Cells were cultured in RPMI 1640 medium containing 1% L-glutamine, 1% sodium pyruvate, 2% Minimum Essential Medium (MEM) non-essential amino acids, 1% penicillin/streptomycin, 0.2% gentamicin (all from Gibco, UK) and 10% fetal calf serum (Sigma, UK).
Experimental protocol
Female BALB/c severe combined immunodeficiency (SCID) mice were obtained from Harlan (Bicester, UK) at 8 weeks of age. Mice were all housed in sterile conditions on vented racks. All procedures were carried out in accordance with the Animals (Scientific Procedures) Act 1986.
Mice were acclimatized for 1 week and then primed with 500 μl intraperitoneally (i.p.) of pristane (2,6,10,14-tetramethylpentadecane; Sigma), which stimulates macrophages to produce growth factors and create an optimal environment for hybridoma cell growth. Ten days later, all mice were injected with the same amount of hybridoma cells (1 × 106 cells in 500 μl of RPMI 1640).
Mice were divided into eight groups of five mice each. Three groups were injected with hybridomas producing antiphospholipid antibodies: IS4 producing human IgG aCL; 12A1-A17.3 producing murine IgG1 anti-β2-GPI; and 16A3-14.11 producing murine IgM aCL [18]. Another three groups were injected with hybridomas secreting antibodies matched for the same isotype and species but that did not have antiphospholipid specificity: TW producing human IgG; 29J3-119 producing murine IgG1; and 16B4-2 producing murine IgM. As negative controls, one group of mice was injected with CBF7, the non-secreting mouse–human heteromyeloma fusion partner cell, and one group was not injected with hybridoma cells.
Throughout the experimental period, proteinuria (Albustix; Bayer Diagnostics, Berkshire, UK) and weight was assessed every 72 h. Mice were killed when the development of ascites resulted in a 20% increase in body weight, which occurred on average 1.2 months after pristane priming. To avoid possible disparities due to the timing of ascites formation, animals in the experimental and control groups were matched by the time they were killed. All investigations, including organ collection for histopathology analysis, were done at the time of killing.
Human IgG enzyme-linked immunosorbent assay (ELISA)
Polystyrene 96-well plates (Maxisorp; Nunc, Roskilde, Denmark) were coated with 2.5 μg/ml of goat anti-human IgG (Sigma) diluted in phosphate-buffered saline (PBS) (pH 7.2). After incubation for 16 h at 4°C, plates were washed three times with PBS and blocked with 1% (w/v) bovine serum albumin (BSA) (Sigma) in PBS for 1 h at 37°C. Serum samples were diluted (1:200) in PBS containing 0.05% Tween 20 (Sigma), and were incubated on the plate for 1 h at 37°C. Following three washes, bound antibodies were detected by incubation for 1 h at 37°C with a 1:1000 dilution of affinity-purified goat anti-human IgG conjugated to alkaline phosphatase conjugate (Sigma) and, following washing, developed with the substrate p-nitrophenol phosphate (Sigma). Optical density was measured at 405nm with a reference filter at 490 nm. The sample concentrations were calculated by reference to the linear portion of a standard curve of purified human IgG (Sigma) run on every plate.
Anti-cardiolipin ELISA (human and murine)
IgG (human) and IgM (murine) aCL were measured by ELISA, using microtitre plates (Polysorp; Nunc, Life Technologies, Paisley, UK), which were coated overnight at 4°C with 50 μg/ml cardiolipin (bovine heart; Sigma-Aldrich, Poole, UK) in 70% ethanol. Blocking was performed with 1% (w/v) BSA (Sigma) in PBS for 1 h at 37°C. Plates were then washed once using PBS. Samples (50μl) (diluted 1:100 for human sera and 1:200 for murine sera), and positive IgG and IgM controls were added to duplicate wells for 1 h at 25°C. After washing three times with PBS, 100μl of alkaline phosphatase-conjugated anti-human IgG and antimouse IgM (both 1:1000 in the blocking agent) were added and incubated for 1 h. p-Nitrophenyl phosphate (100μl) (Sigma) in diethanolamine buffer (pH 9.8) was added; samples were incubated at 37°C for colour development, and the absorbance was read at 405nm after 1 h. Results were expressed as a percentage of the positive control: OD (sample)/OD (positive control) × 100.
aβ2GPI ELISA (murine)
IgG–β2GPI was measured by ELISA, using a commercial kit (Diastat anti-β2GPI; Axis-Shield Diagnostics, Dundee, UK) based on a method previously described [19]. Murine aβ2GPI was assayed by adaptation of the human kit to detect murine antibodies with goat anti-mouse IgG–alkaline phosphatase conjugate (Sigma). Assays were developed as described above.
Nitrotyrosine ELISA
Nitrotyrosine was measured by ELISA using a commercial kit (Hbt nitrotyrosine; HyCult Biotechnology, Uden, Netherlands) in accordance with the instructions of the manufacturer. Standard curves were performed in each plate, using samples with known nitrotyrosine concentrations included in the kit.
Paraoxonase activity
Serum PON activity was measured as described by Eckerson et al. [20] with some modifications. Briefly, paraoxon (1.0 mM), freshly prepared in 300 μl of 50mM glycine buffer containing 1mM calcium chloride (pH 10.5), was incubated at 37°C with 5μl of serum for 15 min in 96-well plates (Polysorp). p-Nitrophenol formation was monitored at 412nm for 15 min to exclude early paraoxon hydrolysis due to irreversible binding of albumin and non-specific esterases. Activity was expressed as μmol p-nitrophenol per ml serum per min.
Total antioxidant capacity (TAC) of plasma
Two different methods assessed the total antioxidant capacity of plasma. The first employed peroxynitrite (ONOO−) quenching: 100μl of phosphate buffer (50mM, pH 7.4) containing Pholasin® (1.7 μg/ml) was pipetted into a microcuvette. Plasma or buffer for control (5 μl) was added. The reaction was initiated by adding 3-morpholino-sydnonimine HCl (SIN-1) (2 μl of 2 mg/ml in water), and light emission was measured continuously at 5 min intervals until the maximum reading was obtained. Antioxidant capacity was expressed as the time at which maximum light was emitted. Lower values reflected a decreased TAC of plasma (peroxynitrite-related).
The second method employed superoxide anion (O2−) quenching: 100 μl of phosphate buffer (50mM, pH 7.4) containing Pholasin (0.5 μg/ml) and adjuvant K (50 μl/ml) was pipetted into a microcuvette, and plasma or buffer for control (5 μl) was added. Next, 5 μl of xanthine (50mM) was added, and the reaction was initiated by the addition of 20 μl of xanthine oxidase (0.5 U/ml phosphate buffer). The signal was recorded over a period of 5 min on a 121 LKB recorder (Bromma, Sweden). Antioxidant capacity was expressed as the counts (mV) at maximum light emission. Higher values reflected a reduced TAC of plasma (superoxide-related).
Serum nitrate and nitrite (NO)
The sum of nitrate and nitrite was determined using the Griess reaction, as previously reported [21]. Briefly, serum was diluted 1:4 with PBS, and 200 μl of this solution was ultrafiltered by centrifugation at 10 000 g for 1 h, using 10 kDa molecular weight filters (Ultrafree-MC; Millipore). Only clear and colourless filtrates were tested. The assay was performed in a standard flat-bottomed 96-well polystyrene microtitre plate, containing 50 μl/well of standard or sample. The assay was blanked against PBS. Fifty microlitres of nitrate reductase and 50μl β-NADPH was added to each well, giving final concentrations of 300 U/l and 25 μmol/l, respectively. The plate was incubated at room temperature for 3 h. Excess β-NADPH was consumed by addition of 50 μl of PBS containing L-glutamic dehydrogenase, α-ketoglutaric acid and NH4Cl (final concentrations 500 U/l, 4 mmol/l and 100 mmol/l, respectively), followed by 10 min of incubation at 37°C. NO concentration was then determined by the addition of 50 μl each of Griess reagents 1 and 2. After incubation for 10 min at room temperature, the absorbance was read at 540 nm.
Haematoxylin and eosin histological stain
Formalin-fixed, paraffin-embedded kidney sections from the SCID mice were stained with haematoxylin and eosin. An experienced histopathologist examined these sections for morphological evidence of vascular damage, thrombosis or other abnormalities. The histopathologist was blind to the mouse treatments.
Western blot analysis for inducible and endothelial nitric oxide synthase
Immunoblot assays were performed according to previously described methods [22] with some modifications. In brief, homogenates from mouse heart were obtained after sonication, and proteins were separated under reducing conditions on 7% polyacrylamide gels. Proteins were then transferred to polyvinylidene difluoride membranes (PVDF; Millipore) and quenched with blocking buffer containing 5% non-fat milk in 0.1% PBS–Tween-20 for 1 h at room temperature. After one wash, membranes were incubated overnight at 4°C with primary antibody [rabbit anti-endothelial western blot analysis (iNOS and eNOS) (eNOS) or rabbit anti-inducible nitric oxide synthase (iNOS); Santa Cruz Biotechnology, USA], 1:1000 in blocking buffer. After several consecutive washes, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (donkey anti-rabbit; Santa Cruz Biotechnology, CA, USA) in blocking buffer for 1 h. Assays were performed using the enhanced chemiluminescence (ECL) detection system (Pharmacia Biotech, Piscataway, NJ, USA).
Immunohistochemistry (iNOS and eNOS)
Paraffin-embedded sections of kidney blood vessels were dewaxed and antigen was retrieved by boiling the sections for 180 s in Tris-EDTA (pH 9.0). Following flushing in tap water, slides were rinsed in 0.05% Tween 20 in Tris-buffered saline (TBS/Tween). Endogenous peroxidase activity was blocked for 30 min using Dako Peroxidase block (Dako, Ely, UK). The sections were rinsed in TBS/Tween and primary antibody was applied for 60 min at room temperature (iNOS and eNOS were diluted 1/100 in TBS). The sections were rinsed in TBS/Tween and EnVision reagent (Dako) was applied for 60 min, then rinsed again in TBS/Tween, and EnVision DAB (Dako) was applied for 10 min. The slides were counterstained with haematoxylin before being dehydrated and mounted.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA). Non-parametric tests were employed to compare differences between groups (Kruskal–Wallis test) and to evaluate associations between variables (Spearman’s rank test). A stepwise multiple regression analysis tested the independence of the associations found by univariate analysis.
Results
Total IgG and IgM antibody levels in mouse groups
The two groups of mice injected with hybridoma-producing human IgG cells (whether antiphospholipid or not) tested positive for human IgG, with no significant difference between groups. Likewise, the four groups of mice injected with hybridoma-producing murine IgG and IgM (whether antiphospholipid or not) tested positive for murine IgG and IgM, with no significant difference between groups (Table 1).
Table 1.
Characterization of oxidative status: PON activity, TAC (peroxynitrite and superoxide quenching), NO and nitrotyrosine in the different animal groups
| Hybridoma | Origin | Antibody and relative concentration (OD) | PON (μmol/ml/min) | TAC (perox) (min) | TAC (superox) (mV) | NO (μmol/l) | NT (μmol/l) |
|---|---|---|---|---|---|---|---|
| IS4 | Human | IgG aCL, 1.35±0.3 | 74±4* | 27±2* | 4.0±0.2* | 36.9±8.9* | 1.50±0.06* |
| TW | Human | IgG control, 1.22±0.2 | 88±4 | 45±7 | 1.9±0.3 | 93.2±35.5 | 1.25±0.15 |
| 12A1-A17.3 | Murine | IgG aβ2-GPI, 1.05±0.2 | 75±5 | 24±2** | 2.3±0.8 | 110.9±31.5 | 1.29±0.08 |
| 16A3-14.11 | Murine | IgM aCL, 0.95±0.2 | 91±4 | 29±2*** | 2.2±0.2 | 94.3±19.2 | 1.30±0.04 |
| 29J3-119 | Murine | IgG control, 1.42±0.4 | 81±10 | 34±9 | 2.3±0.2 | 102.9±35.4 | 1.28±0.11 |
| 16B4-2 | Murine | IgM control, 1.13±0.4 | 85±2 | 36±9 | 2.3±0.2 | 107.3±18.3 | 1.34±0.06 |
| CBF7 | NA | NA | 90±10 | 37±4 | 2.3±0.2 | 97.5±12.4 | 1.36±0.04 |
| No hybridoma | NA | NA | 101±6 | 45±4 | 1.9±0.4 | 68.9±7.7 | 1.34±0.07 |
Significant compared with TW, CBF7 and ‘no cells’ groups.
Significant compared with 29J3-119, CBF7 and ‘no cells’ groups.
Significant compared with 16B4-2 and ‘no cells’ groups.
Antibody concentration results reflect aCL and aβ2-GPI antibodies in the respective groups; results in the murine control groups reflect total IgG and IgM antibody concentrations.
NA, not applicable; OD, optical density (% of positive control); TAC, total antioxidant capacity; NO, nitric oxide; perox, peroxynitrite; superox, superoxide; NT, nitrotyrosine.
Antiphospholipid antibody levels in mouse groups
Animals injected with hybridomas producing mouse aCL and aβ2-GPI tested positive for both antibodies (Table 1). None of the animals in the control groups (TW, 29J3-119, 16B4-2, CBF7, or ‘no cells’) had detectable levels of human aCL, murine aβ2-GPI or murine aCL antibodies in their sera.
Characterization of oxidative status
PON activity
Values of all the oxidative status variables are shown in Table 1. PON activity was reduced in the IS4 group (human IgG aCL) when compared with its matched control TW (human IgG), CBF7 and the ‘no cells’ groups (P always <0.01).
Mice positive for murine IgG aβ2-GPI showed decreased PON activity compared with the matched murine control group, but the difference did not reach statistical significance. There were no differences regarding PON activity in the remaining groups.
TAC (peroxynitrite quenching)
TAC assessed by peroxynitrite quenching was low in the IS4 group (human IgG aCL), when compared with the human IgG control, with CBF7 and with the ‘no cells’ groups (P<0.01, P<0.01 and P<0.008, respectively). TAC was also significantly low in mice injected with hybridomas producing murine IgG aβ2-GPI and IgM aCL when compared with the respective control group (irrelevant murine IgG or IgM) (P<0.03 and P<0.05, respectively).
TAC (superoxide quenching)
TAC assessed by superoxide quenching was decreased in the IS4 (human IgG aCL) group, when compared with the human IgG control, CBF7 and ‘no cells’ controls (P always <0.01) It should be noted that, in the case of superoxide quenching, reduced superoxide quenching is reflected by higher values of plasma TAC. All other groups had similar levels.
NO and nitrotyrosine
NO (sum of nitrate and nitrite) was decreased in the IS4 group (human IgG aCL) when compared with the control groups (P<0.04). In contrast, there was no significant difference between the groups positive for murine IgG aβ2-GPI or IgM aCL and their relevant control groups. Nitrotyrosine was found to be elevated only in the IS4 group (human IgG aCL). This was significant when compared with all the control groups (P<0.03).
Relationship between antioxidant variables
PON activity showed an inverse correlation with both total human IgG titres (P<0.001) and TAC (superoxide) (P<0.008) in mice injected with human IgG-producing hybridomas. There was also a direct correlation between PON activity and TAC assessed as peroxynitrite quenching (P<0.0009). NO levels were inversely correlated with TAC assessed as both superoxide quenching (P<0.008) and nitrotyrosine levels (P<0.01).
Multiple regression models
To evaluate factors that could independently predict PON activity, NO levels and nitrotyrosine, stepwise multiple regressions were performed and the following variables were considered: antibody type, total IgG, total IgM, and TAC (peroxynitrite and superoxide).
TAC (peroxynitrite) and total IgG levels were independent predictors of PON activity [P<0.0009 for TAC (peroxynitrite) and P<0.02 for total IgG titre]. TAC (superoxide) was the only independent predictor for NO levels (P<0.008) and nitrotyrosine (P<0.002).
Western blot analysis (iNOS and eNOS)
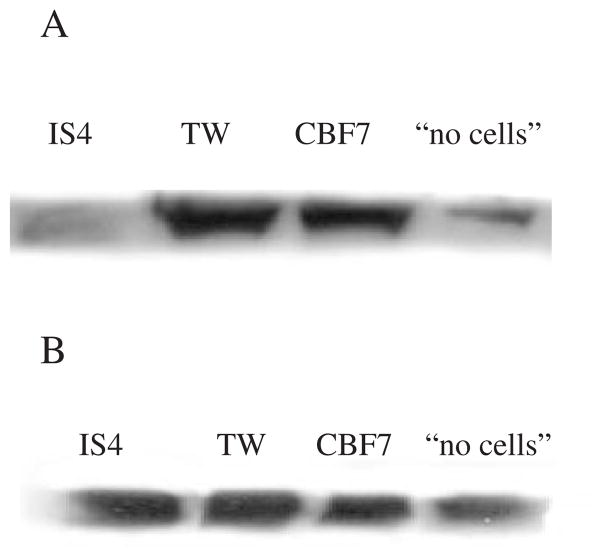
Expression of iNOS in the hearts of mice that were inoculated with the hybridoma producing IS4 (human IgG aCL) was practically abolished compared with heart samples from control groups; that is, mice inoculated with the hybridoma producing the human IgG and mice inoculated with the hybridoma cells alone (CBF7). The expression of iNOS was also low in the non-inoculated group of mice: these mice did not develop the peritonitis necessary to support the growth of the hybridomas. The other control groups that developed hybridoma-induced peritonitis all showed strong iNOS expression (Fig. 1A). The expression of iNOS in the murine Ig groups showed no differences (not shown). There were no significant differences in the expression of eNOS in heart tissues collected from both the human (Fig. 1B) and the murine (not shown) Ig groups.
Fig. 1.
(A and B) Western blot analysis of iNOS (A) and eNOS (B) in the heart of animals injected with IS4, TW and CBF7 and ‘no cells’ controls. Mice from the ‘no cells’ group did not have cell-induced peritonitis, hence the decreased iNOS expression when compared with the other control groups.
Histology and immunohistochemistry (iNOS and eNOS)
Haematoxylin/eosin staining of tissue sections from heart, kidney, lung and brain showed no evidence of fibrin deposition, vascular damage or thrombosis.
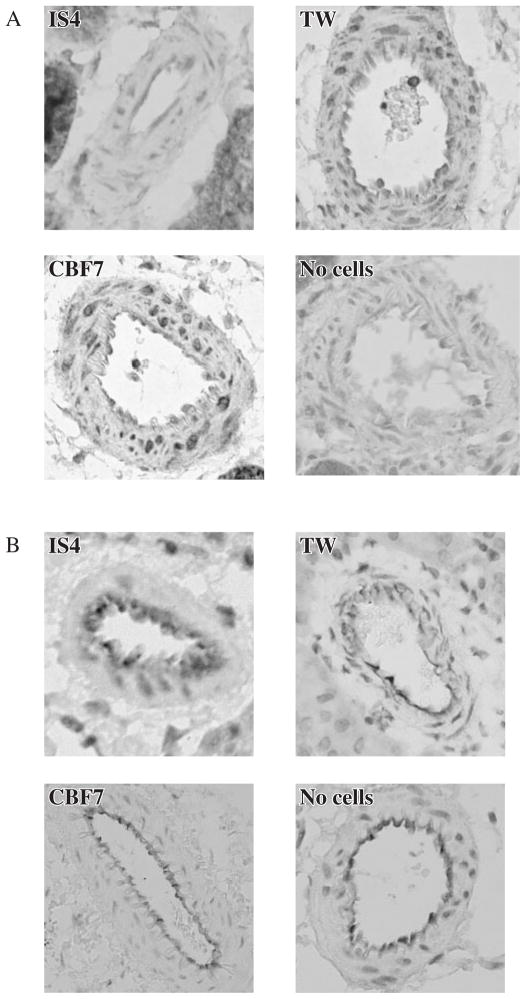
Immunostaining for iNOS was markedly decreased in vessels of mice that received the IS4-producing hybridoma when compared with the relevant control groups (Fig. 2A). In contrast, there were no evident changes in staining for eNOS in any of these groups (Fig. 2B).
Fig. 2.
(A and B) Immunohistochemistry for iNOS (A) and eNOS (B) in the kidney vessels of mice injected with IS4, TW and CBF7 and ‘no cells’ controls.
Discussion
The study demonstrates that human IgG aCL generated from hybridoma cells induces a reduction in PON activity in SCID mice compared with the relevant IgG control group. The reduction in PON induced by the murine IgG aβ2-GPI was not significant, probably reflecting differences in the affinity, avidity and fine specificity of this antibody for PON. Given the low pathogenic potential of IgM aCL, we almost expected that murine IgM aCL did not affect PON activity. These results are in keeping with our human data on primary APS, in which both IgG aCL and aβ2-GPI titres were inversely related to decreased PON activity [16].
TAC represents the capacity of plasma to resist oxidation. One of the major oxidative pathways involves the generation of superoxide and its subsequent reaction with NO to form peroxynitrite [23]. Peroxynitrite can oxidize different molecules, including lipids and lipoproteins [24], and its persistent generation allows the formation of nitrotyrosine moieties on proteins, which may represent a marker of ongoing nitrosative stress [25].
TAC is a good indicator of oxidative potential at any given time. By the peroxynitrite quenching method, all mice groups injected with hybridomas secreting aCL (human or murine) or aβ2-GPI (murine) antibodies showed reduced TAC, suggesting a link between aCL and peroxynitrite formation. Since PON prevents peroxynitrite-mediated lipid peroxidation [26], a reduction of PON activity, as seen in the IgG aCL and aβ2-GPI groups, contributes to increased oxidative stress. The superoxide quenching method provides further evidence for a pro-oxidant tendency, in that higher values of TAC were related to increased level of plasma superoxide in the IS4 antibody group.
Mice and humans with SLE generate significant amounts of NO [27, 28], this has never been related to aCL antibodies, whereas in primary APS low NO inversely correlated to IgG aCL [14]. Here we confirm the previous observation, since plasma NO level was lower in the group injected with IS4-producing hybridoma cells.
IgG aCL (IS4, in particular) can activate endothelial cells [29], hence one might have anticipated increased NO production, due to increased expression of iNOS. Instead, western blotting and immunohistochemistry revealed reduced iNOS expression, with no significant differences in the expression of eNOS, consistent with reduced generation of NO.
The high levels of nitrotyrosine found in the IS4 group may explain this discrepancy. Because nitrotyrosine indicates nitrosative stress in vivo [25, 30], its elevation may indicate previous elevated endothelial NO production induced by aCL. In an oxidative environment NO is shifted towards peroxynitrite formation, the latter inducing down-regulation of iNOS. If NO was not generated at all, there would not be any measurable peroxynitrite. On the other hand, down-regulation of iNOS expression may follow a period of increased activity, as reported, but not in autoimmune diseases [31]. The expression of iNOS and eNOS is controlled by different feedback mechanisms, the most important of which relates to NO itself [32]. Increased local levels of NO down-regulate iNOS by inhibiting the transcriptional factor NF-κβ in macrophages and endothelial cells [33], causing lowered NO production [34, 35]. A by-product of lipid peroxidation, 4-hydroxynonenal, inhibits NF-κβ activation and consequent iNOS expression [36], whereas IgG aCL induces NF-κβ in endothelial cells [37]. An alternative explanation would be that in the face of iNOS down-regulation eNOS still provides a source of NO to scavenge superoxide.
In conclusion, we replicated in a mouse model our findings from APS patients [16]. Whereas in humans it is difficult to state when these processes begin, in the mouse model they appear as early as 30 days. For this very reason we could not detect any (micro)thrombosis or vascular damage. A diet enriched in cholesterol would have been necessary to favour the appearance of atherosclerotic changes in this short span of time, but increased oxidation induced by hypercholesterolaemia may have confounded our results and a second hit would have been required to induce thrombosis. Nevertheless, IgG aPL may induce in mice a sequence of decreased PON activity, enhanced peroxynitrite formation and inhibition of iNOS expression. This is the first report to show intimate relationships between oxidative/nitrosative pathways and IgG aPL; these could be explored in more suitable models to test their association with vascular manifestations of APS.
Key messages.
This study shows an association between anticardiolipin antibodies, paraoxonase inhibition and a decrease in iNOS expression.
These effects induce a pro-oxidant environment, which can explain in Rheumatology part the enhanced atherogenesis and thrombosis found in antiphospholipid syndrome.
Footnotes
The authors have declared no conflicts of interest.
References
- 1.Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346;10:752–63. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 2.Hughes GRV, Harris EN, Gharavi AE. The anticardiolipin syndrome. J Rheumatol. 1986;13:486–9. [PubMed] [Google Scholar]
- 3.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Antiphospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci USA. 1990;87:4120–4. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuura E, Igarashi Y, Yasuda T, Triplett DA, Koike T. Anticardiolipin antibodies recognize beta 2-glycoprotein I structure altered by interacting with an oxygen modified solid phase surface. J Exp Med. 1994;179:457–62. doi: 10.1084/jem.179.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntyre JA, Wagenknecht DR, Sugi T. Phospholipid binding plasma proteins required for antiphospholipid antibody detection— an overview. Am J Reprod Immunol. 1997;37:101–10. doi: 10.1111/j.1600-0897.1997.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 6.Rampazzo P, Biasiolo A, Garin J, et al. Some patients with antiphospholipid syndrome express hitherto undescribed antibodies to cardiolipin-binding proteins. Thromb Haemost. 2001;85:57–62. [PubMed] [Google Scholar]
- 7.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 8.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 9.Lelamali K, Wang W, Gengaro P, Edelstein C, Schrier RW. Effects of nitric oxide and peroxynitrite on endotoxin-induced leukocyte adhesion to endothelium. J Cell Physiol. 2001;188:337–42. doi: 10.1002/jcp.1128. [DOI] [PubMed] [Google Scholar]
- 10.Darley-Usmar VM, Hogg N, O’Leary VJ, Wilson MT, Moncada S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Radic Res Commun. 1992;17:9–20. doi: 10.3109/10715769209061085. [DOI] [PubMed] [Google Scholar]
- 11.Ames PR, Nourooz-Zadeh J, Tommasino C, Alves J, Brancaccio V, Anggard EE. Oxidative stress in primary antiphospholipid syndrome. Thromb Haemost. 1998;79:447–9. [PubMed] [Google Scholar]
- 12.Morrow JD, Roberts LJ. The isoprostanes. Current knowledge and directions for future research. Biochem Pharmacol. 1996;51:1–9. doi: 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- 13.Iuliano L, Pratico D, Ferro D, et al. Enhanced lipid peroxidation in patients positive for antiphospholipid antibodies. Blood. 1997;90:3931–5. [PubMed] [Google Scholar]
- 14.Ames PR, Tommasino C, Alves J, et al. Antioxidant susceptibility of pathogenic pathways in subjects with antiphospholipid antibodies: a pilot study. Lupus. 2000;9:688–95. doi: 10.1191/096120300677692516. [DOI] [PubMed] [Google Scholar]
- 15.Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:473–80. doi: 10.1161/01.atv.21.4.473. [DOI] [PubMed] [Google Scholar]
- 16.Delgado Alves J, Ames PR, Donohue S, et al. Antibodies to high-density lipoprotein and beta2-glycoprotein I are inversely correlated with paraoxonase activity in systemic lupus erythematosus and primary antiphospholipid syndrome. Arthritis Rheum. 2002;46:2686–94. doi: 10.1002/art.10542. [DOI] [PubMed] [Google Scholar]
- 17.Zhu M, Olee T, Le DT, Roubey RA, Hahn BH, Woods VL, Jr, Chen PP. Characterization of IgG monoclonal anti-cardiolipin/anti-beta2GPI antibodies from two patients with antiphospholipid syndrome reveals three species of antibodies. Br J Haematol. 1999;105:102–9. [PubMed] [Google Scholar]
- 18.Price BE, Rauch J, Shia MA, et al. Anti-phospholipid autoantibodies bind to apoptotic, but not viable, thymocytes in a beta 2-glycoprotein I-dependent manner. J Immunol. 1996;157:2201–8. [PubMed] [Google Scholar]
- 19.McNally T, Purdy G, Mackie IJ, Machin SJ, Isenberg DA. The use of an anti-beta 2-glycoprotein-I assay for discrimination between anticardiolipin antibodies associated with infection and increased risk of thrombosis. Br J Haematol. 1995;91:471–3. doi: 10.1111/j.1365-2141.1995.tb05324.x. [DOI] [PubMed] [Google Scholar]
- 20.Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983;35:1126–38. [PMC free article] [PubMed] [Google Scholar]
- 21.Giovannoni G, Land JM, Keir G, Thompson EJ, Heales SJR. Adaptation of the nitrate reductase and Griess reaction methods for the measurement of serum nitrate plus nitrite levels. Ann Clin Biochem. 1997;34:193–8. doi: 10.1177/000456329703400212. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Muijsers RB, Folkerts G, Henricks PA, Sadeghi-Hashjin G, Nijkamp FP. Peroxynitrite: a two-faced metabolite of nitric oxide. Life Sci. 1997;60:1833–45. doi: 10.1016/s0024-3205(96)00651-0. [DOI] [PubMed] [Google Scholar]
- 24.Beckman JS, Crow JP. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans. 1993;21:330–4. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- 25.Inoue H, Hisamatsu K, Ando K, Ajisaka R, Kumagai N. Determination of nitrotyrosine and related compounds in biological specimens by competitive enzyme immunoassay. Nitric Oxide. 2002;7:11. doi: 10.1016/s1089-8603(02)00005-8. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed Z, Ravandi A, Maguire GF, et al. Multiple substrates for paraoxonase-1 during oxidation of phosphatidylcholine by peroxynitrite. Biochem Biophys Res Commun. 2002;290:391–6. doi: 10.1006/bbrc.2001.6150. [DOI] [PubMed] [Google Scholar]
- 27.Weinberg JB, Granger DL, Pisetsky DS, et al. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L-arginine. J Exp Med. 1994;179:651–60. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belmont HM, Levartovsky D, Goel A, et al. Increased nitric oxide production accompanied by the up-regulation of inducible nitric oxide synthase in vascular endothelium from patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:1810–6. doi: 10.1002/art.1780401013. [DOI] [PubMed] [Google Scholar]
- 29.Del Papa N, Guidali L, Spatola L, et al. Relationship between antiphospholipid and anti-endothelial cell antibodies III: beta 2 glycoprotein I mediates the antibody binding to endothelial membranes and induces the expression of adhesion molecules. Clin Exp Rheumatol. 1995;13:179–85. [PubMed] [Google Scholar]
- 30.Oates JC, Christensen EF, Reilly CM, Self SE, Gilkeson GS. Prospective measure of serum 3-nitrotyrosine levels in systemic lupus erythematosus: correlation with disease activity. Proc Assoc Am Physicians. 1999;111:611–21. doi: 10.1046/j.1525-1381.1999.99110.x. [DOI] [PubMed] [Google Scholar]
- 31.Vallance P, Collier J, Bhagat K. Infection, inflammation, and infarction: does acute endothelial dysfunction provide a link? Lancet. 1997;349:1391–2. doi: 10.1016/S0140-6736(96)09424-X. [DOI] [PubMed] [Google Scholar]
- 32.Assreuy J, Cunha FQ, Liew FY, Moncada S. Feedback inhibition of nitric oxide synthase activity by nitric oxide. Br J Pharmacol. 1993;108:833–7. doi: 10.1111/j.1476-5381.1993.tb12886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Connelly L, Palacios-Callender M, Ameixa C, Moncada S, Hobbs AJ. Biphasic regulation of NF-kappa B activity underlies the pro- and anti-inflammatory actions of nitric oxide. J Immunol. 2001;166:3873–81. doi: 10.4049/jimmunol.166.6.3873. [DOI] [PubMed] [Google Scholar]
- 34.Swierkosz TA, Mitchell JA, Warner TD, Botting RM, Vane JR. Co-induction of nitric oxide synthase and cyclo-oxygenase: interactions between nitric oxide and prostanoids. Br J Pharmacol. 1995;114:1335–42. doi: 10.1111/j.1476-5381.1995.tb13353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheffler LA, Wink DA, Melillo G, Cox GW. Exogenous nitric oxide regulates IFN-gamma plus lipopolysaccharide-induced nitric oxide synthase expression in mouse macrophages. J Immunol. 1995;155:886–94. [PubMed] [Google Scholar]
- 36.Hattori Y, Hattori S, Kasai K. 4-hydroxynonenal prevents NO production in vascular smooth muscle cells by inhibiting nuclear factor-kappaB-dependent transcriptional activation of inducible NO synthase. Arterioscler Thromb Vasc Biol. 2001;21:1179–83. doi: 10.1161/hq0701.092135. [DOI] [PubMed] [Google Scholar]
- 37.Meroni PL, Raschi E, Camera M, et al. Endothelial activation by aPL: a potential pathogenetic mechanism for the clinical manifestations of the syndrome. J Autoimmun. 2000;15:237–40. doi: 10.1006/jaut.2000.0412. [DOI] [PubMed] [Google Scholar]