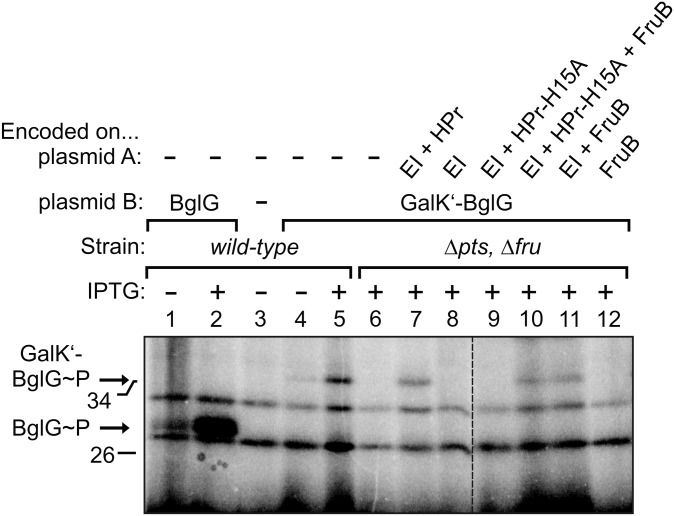
Fig. 2.
BglG becomes phosphorylated in vivo also in the absence of BglF, and this phosphorylation requires EI and either HPr or its paralog FruB, but not EIIAGlc. Phosphorylation of BglG or the GalK′–BglG fusion protein was addressed in various genetic backgrounds by metabolic labeling of proteins with H3[32P]PO4. The wild type (strain R1279; lanes 1–5) and the double mutant lacking the chromosomal ptsHIcrr and fruBKA operons (strain R1969; lanes 6–12) were tested. Both strains lacked the natural bgl operon. With exception of lane 3, the strains harbored a p15A plasmid (plasmid B), which carried either bglG (lanes 1 and 2; plasmid pFDX2942) or the galK′–bglG fusion gene (lanes 4–12; plasmid pFDX3225) under control of the IPTG-inducible Ptac promoter. In lanes 7–12, the ΔptsHIcrr ΔfruBKA mutant was complemented with additional compatible pSC101-type plasmids (plasmid A), which expressed the PTS proteins as indicated from a constitutive promoter. These plasmids were pFDX4735 (lane 7), pFDX4733 (lane 8), pFDX4736 (lane 9), pFDX4738 (lane 10), pFDX4737 (lane 11), and pFDX4739 (lane 12). Lac repressor was delivered from the ColEI-type plasmid pFDY226. The transformants were grown in minimal medium containing glycerol and 100 μM IPTG for induction of Ptac-controlled genes as indicated. The dashed line indicates cropping of one lane from the original autoradiograph.