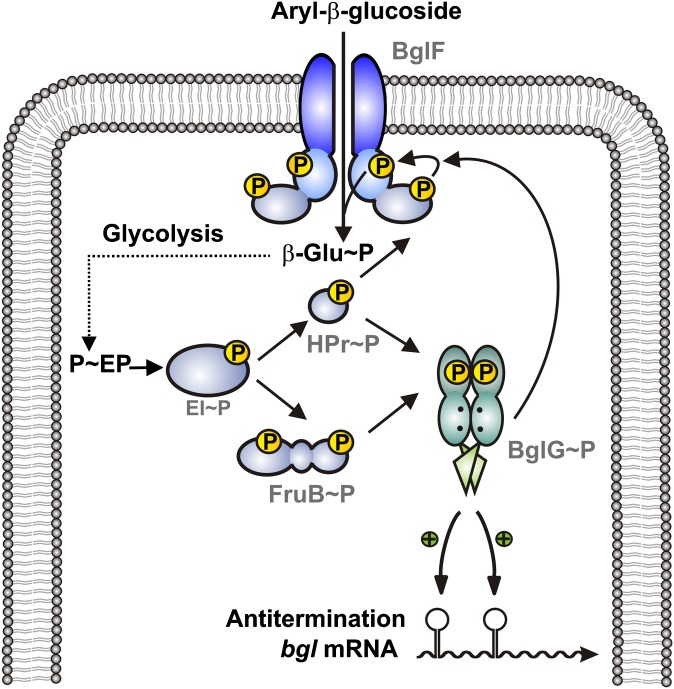
Fig. 5.
Model for activation of the BglG antiterminator protein by the PTS. BglG requires two distinct (de)phosphorylation events to gain activity. On the one hand, BglG must be dephosphorylated by the β-glucoside transporter BglF, which only occurs when BglF is engaged in transport (5, 6). In addition to this dephosphorylation, which according to previous data occurs in domain PRD1 of BglG (9), BglG requires phosphorylation at a different site to become active (ref. 16) and this work). This phosphorylation is directly catalyzed by HPr, and its paralog FruB can substitute HPr in this reaction (this work). In vivo (9) and biochemical evidence (this work) suggests that this activating phosphorylation takes place at residue His208 in PRD2. According to previous analysis (16), it serves as a carbon catabolite mechanism down-regulating BglG activity, when other PTS sugars become available in addition to β-glucosides. Transport of these additional PTS sugars drains phosphoryl groups from HPr, leading to a smaller population of active BglG molecules.