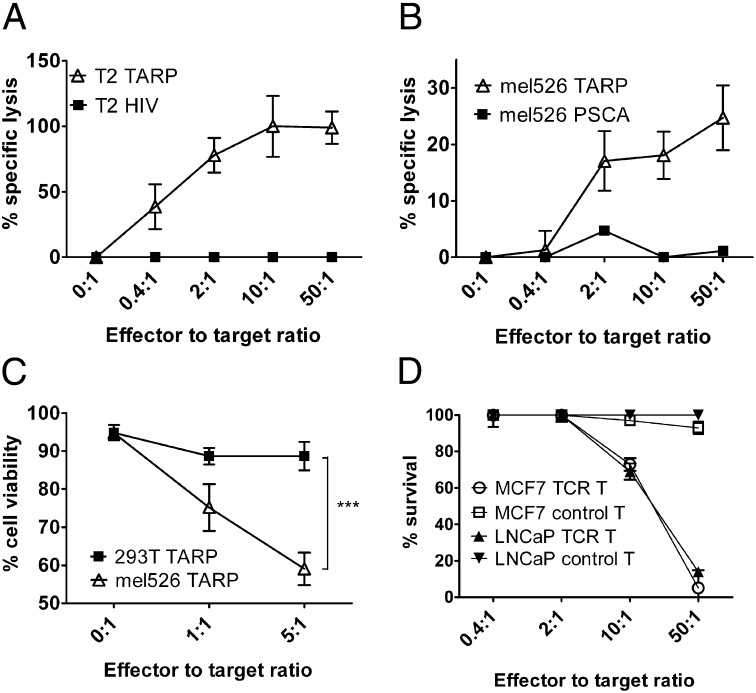
Fig. 4.
TARP-TCR–modified T cells specifically kill TARP4–13-presenting tumor cells. (A) TARP-TCR–engineered T cells were mixed with 51Cr-labeled T2 target cells, pulsed either with the HLA-A2–restricted TARP(P5L)4–13 peptide (T2 TARP) or the irrelevant HLA-A2–restricted HIV-1pol476–484 peptide (T2 HIV). (B) TARP-TCR–engineered T cells were mixed with 51Cr-labeled mel526 target cells transfected with a plasmid encoding either the full-length wild-type TARP (mel526 TARP) or the irrelevant PSCA protein (mel526 PSCA). Specific killing was assayed in terms of 51Cr release from dead target cells after 4 h. Data represent triplicate samples from one representative experiment of two. (C) TARP-TCR–engineered T cells were mixed with either HLA-A2+ target cells (mel526) or HLA-A2− target cells (HEK-293T) transfected with a plasmid encoding the full-length wild-type TARP protein. Target cell viability was assayed by propidium-iodide staining of CD3− target cells after overnight coculture with TARP-TCR–engineered T cells. Asterisks denote significant difference (***P < 0.001, two way ANOVA). Shown is pooled data from two experiments run in triplicates. (D) The HLA-A2–engineered prostate cancer cell line LNCaP and the IFN-γ pretreated breast cancer cell line MCF7 were transduced with a lentiviral vector encoding firefly luciferase. TARP-TCR T cells or nontransduced control T cells were cocultured for 72 h with luciferase-expressing LNCaP or MCF7 target cells at ratios 0.4:1, 2:1, 10:1, and 50:1. Luciferase expression was then examined as a measure of target cell viability. Luciferase expression from target cells not exposed to T cells was set to 100%.