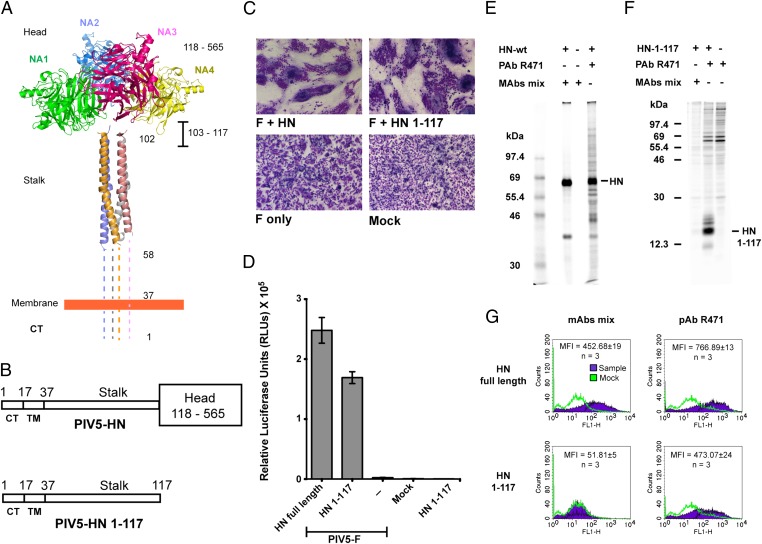
Fig. 1.
The PIV5 HN stalk domain is sufficient to activate F and mediate cell–cell fusion. (A) Structural view of the PIV5 HN full-length protein based on X-ray crystal structures of the tetrameric PIV5 HN globular head domains (16) and the 4HB of the PIV5 HN stalk domain (21). Regions for which there is no known structure (amino acids 1–58 and 103–117) are represented by dotted lines. CT, cytoplasmic tail; NA1–NA4, globular neuraminidase head domains. (B) Schematic representation of the PIV5 HN full-length WT protein (amino acids 1–565) (Upper) and the PIV5 HN stalk protein (amino acids 1–117) lacking the globular head domain (amino acids 118–565) (Lower). CT, cytoplasmic tail; TM, transmembrane domain. (C) Representative micrographs of syncytia showing cell–cell fusion in BHK-21 cells transfected with F and WT HN or HN 1–117 stalk 18 h posttransfection. (D) Luciferase reporter assay of cell–cell fusion. (E and F) Radioimmunoprecipitation of [35S]-TranS–labeled transfected 293T cell lysates, using PIV5 HN pAb R471 or a mixture of mAbs to the PIV5 HN globular head. (E) WT HN protein analyzed on a 10% (wt/vol) SDS/PAGE gel. (F) HN 1–117 stalk protein analyzed on a 15% (wt/vol) SDS/PAGE gel. Numbers to the left of each panel are molecular masses in kDa. (G) Representative graphs showing the detection of proteins at the cell surface of 293T cells transfected with WT HN or HN 1–117 stalk using flow cytometry. Surface proteins were detected using a mixture of HN mAbs or HN pAb R471. Average mean fluorescence intensities (MFI) across three independent experiments are shown.