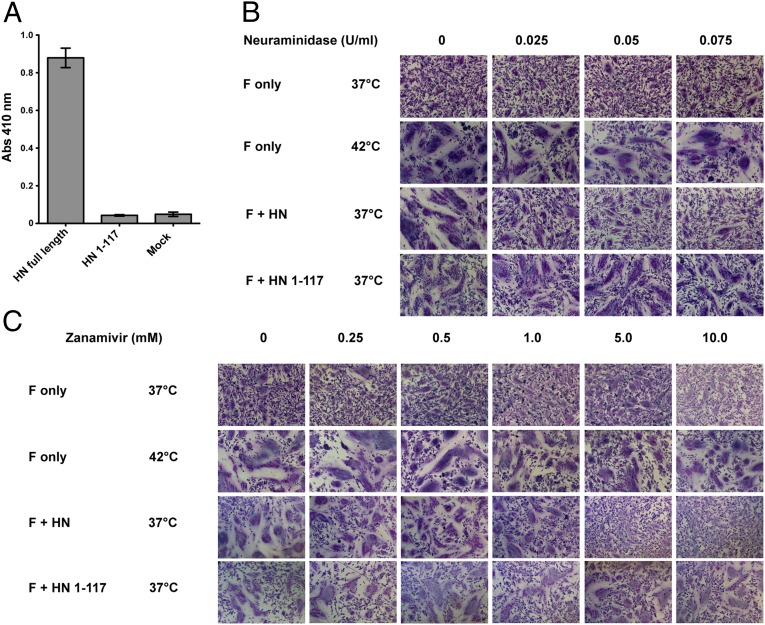
Fig. 4.
F activation mediated by the PIV5 HN 1–117 stalk does not depend on engagement of the sialic acid receptor. (A) Receptor-binding activity of the PIV5 HN 1–117 stalk. Chicken RBCs bound onto surfaces of transfected 293T cells were quantified by lysing the bound RBCs after extensive PBS washes and measuring absorbance of chicken hemoglobin at 410 nm. (B and C) BHK-21 cells transfected with PIV5 F only or cotransfected with PIV5 F and PIV5 HN or PIV5 HN 1–117 stalk were incubated with C. perfringens neuraminidase or zanamivir at 37 °C or 42 °C. Cells were fixed, stained, and imaged 18 h posttransfection. (B and C) Fusion activation in the presence of 0 U/mL, 0.025 U/mL, 0.05 U/mL, and 0.075 U/mL C. perfringens neuraminidase to remove most sialic acid linkages (B) and with the addition of 0 mM, 0.25 mM, 0.5 mM, 1 mM, 5 mM, and 10 mM zanamivir to inhibit PIV5 HN catalytic sites specifically (C).