Table 1.
Calculated and experimental* thermodynamic hydricities (kcal/mol) in CH3CN solution
| Hydride donor | Conjugate hydride acceptor | Expt. |  |
 |
| CHO- | CO | −10.0 | −10.8 | |
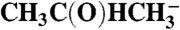 |
CH3COCH3 | 18.5 | 19.8 | |
| [Re(pbnHH•-)(CO)3(Cl)]- | [Re(pbnH•)(CO)3(Cl)]0 | 32.4 | 34.9 | |
| [RuII(bpy)(tpy)(H)]+ | [RuII(bpy)(tpy)(NCMe)]2+ | 39† | 36.0 | 38.7 |
| HCOO- | CO2 | 43 | 39.1 | 42.1 |
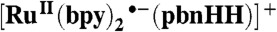 |
[RuII(bpy)2(pbnH•)]2+ | 46.6 | 50.1 | |
 |
[RuII(bpy)2(iso-pbnH•)]2+ | 47.1 | 50.7 | |
| [Cp∗ ReI(NO)(CO)(CHO)]0 | [Cp∗ ReI(NO)(CO)2]+ | 52.6 | 49.7 | 53.4 |
| MNAH | MNA+ | 52.0 | 55.8 | |
| [CpReI(NO)(CO)(CHO)]0 | [CpReI(NO)(CO)2]+ | 55 | 52.8 | 56.8 |
| BNAH | BNA+ | 59 | 53.3 | 57.2 |
| p - monohydroquinone- | p-benzoquinone | 58.9 | 63.3 | |
| H2 | H+ | 76 | 61.2 | 65.8 |
| [Re(pbnHH)(CO)3(Cl)]0 | [Re(pbnH+)(CO)3(Cl)]+ | 76.5 | 82.2 | |
| CH3C(OH)HCH3 |  |
81.6 | 87.7 | |
| [RuII(bpy)2(pbnHH)]2+ | [RuII(bpy)2(pbnH+)]3+ | 82.6 | 88.7 | |
| [RuII(bpy)2(iso-pbnHH)]2+ | [RuII(bpy)2(iso-pbnH+)]3+ | 85.2 | 91.5 | |
| Ph3CH | Ph3C+ | 99 | 92.0 | 98.9 |
