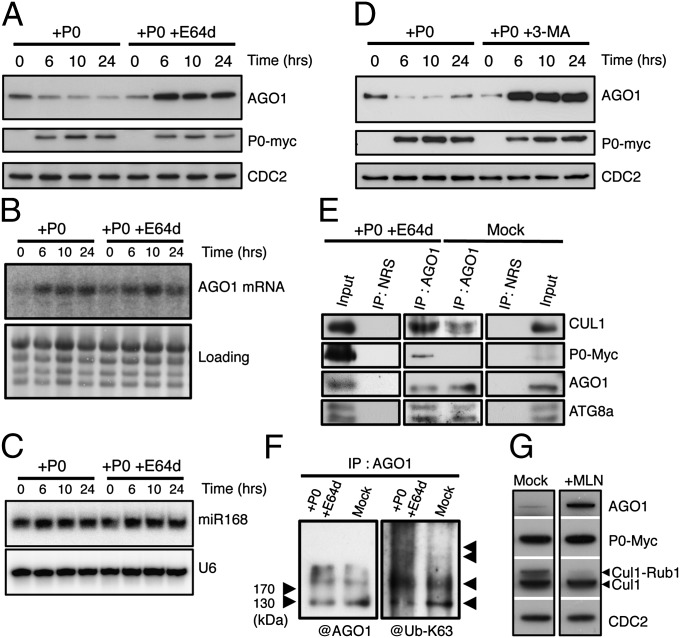
Fig. 1.
P0-mediated degradation of AGO1 is blocked by autophagy inhibitors. AGO1 degradation kinetics were performed on 7-d-old XVE-P0BW-myc seedlings treated with β-estradiol (5 μM) for P0-myc induction. Autophagy was inhibited in its last steps using E64d (20 μM) (A) and AGO1, P0-myc, and CDC2 (loading control) protein accumulation levels were assayed by Western blot on a 24-h period. In a similar manner, AGO1 mRNA (B) and miR168 accumulation (C) was assayed by Northern blot analyses along P0-mediated degradation of AGO1 in presence or in absence of E64d (20 μM). Loading controls are methylene blue staining of the membrane for mRNA and U6 for small RNA blots. (D) Autophagy was inhibited in its first steps using the specific PI-3-kinase class III inhibitor 3-MA (5 mM) and AGO1, P0-myc, and CDC2 (loading control) protein accumulation levels were assayed by Western blot on a 24-h period. (E) Coimmunoprecipitation of AGO1 and SCFP0. XVE-P0BW-myc seedlings were treated with β-estradiol (5 μM) for P0-myc induction and E64d (20 μM) for at least 6 h before protein extraction. Plant extract were immunoprecipitated with an anti-AGO1 antibody and with normal rabbit serum (NRS). IP fractions were submitted to Western blot analysis using antibodies raised against the myc tag for P0 detection and against CUL1, AGO1, and ATG8a. (F) Ubiquitylation status of AGO1 was determined by Western blot analysis of IP fractions using an antibody specifically raised against K63-Ub. (G) Inhibition of SCF activity prevents P0-mediated degradation of AGO1. Seven-day-old XVE-P0BW-myc seedlings were pretreated with MLN-4924 (25 μM) for 3 h before P0-myc induction with β-estradiol (5 μM). The accumulation level of AGO1, P0-myc, CUL1, and CDC2 (loading control) was assayed by Western blot 24 h after P0 induction. Anti-CUL1 antibody detects two bands, the upper one corresponding to the NEDD8/RUB1-modified form of CUL1.