Table 2.
Coupling of Aryl Halides with Secondary Alcohols[a]
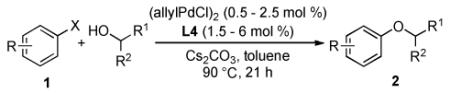
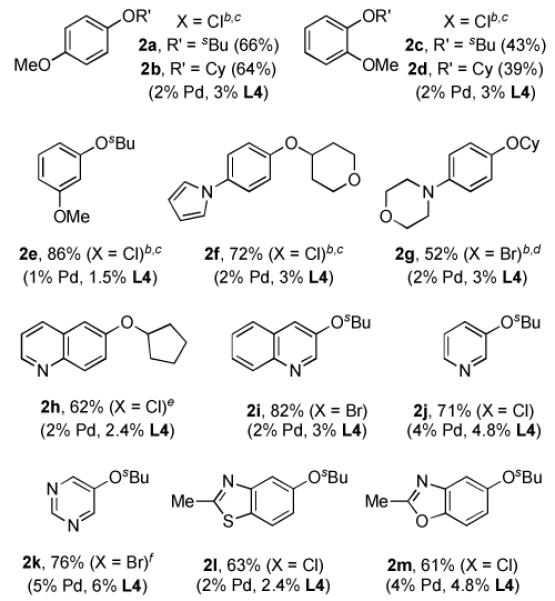
|
Reaction conditions: ArX (1 mmol), alcohol (2 mmol), Cs2CO3 (1.5 mmol), (allylPdCl)2 (0.5 – 2.5 mol %), L4 (1.5 – 6 mol %), toluene (1 mL), 90 °C, 21 h; isolated yields (average of two or more runs).
200 mg of 4Å molecular sieves was added.
in Bu3N.
cyclohexanol (1.5 mmol) was used.
In Et3N.
24 h.
