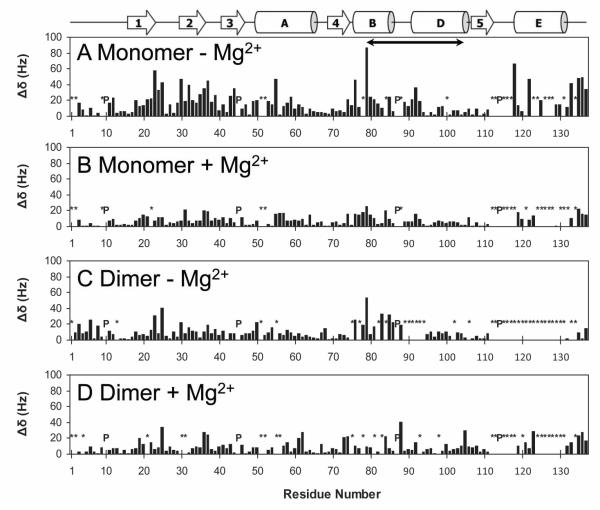
Figure 4.
NMR chemical shift perturbation induced by BHMP07 interaction with the RNH monomer in the absence (A) or presence (B) of 20 mM Mg2+; and with the RNH dimer in the absence (C) or presence (D) of 20 mM Mg2+. Normalized, quadratically weighed 1H, 15N backbone amide resonance shifts induced by BHMP07 (Δδ, cf. Eq. 1) are shown relative to sequence residue. Prolines (P) and backbone NH amide groups whose chemical shift changes could not be tracked (*) are indicated. A schema of secondary structure elements (26) is included for easy reference; the “substrate-handle region” (58) is denoted by a solid arrow (residues 80 to 103). Data were recorded at 600 MHz (1H), at 20°C and pH 7.0.