Abstract
Background
One of the major determinants for survival of severely burned patients is appropriate fluid resuscitation. At present, fluid resuscitation is calculated based on bodyweight or body surface area, burn size, and urinary output. However, recent evidence suggests that fluid calculation is inadequate and that over- and under-resuscitation is associated with increased morbidity and mortality. We hypothesize that optimizing fluid administration during the critical initial phase using a transcardiopulmonary thermo-dilution monitoring device (PiCCO) would have beneficial effects on the outcome of burned patients.
Methods
A cohort of seventy-six severely burned pediatric patients with burns over 30% total body surface area (TBSA) who received adjusted fluid resuscitation using the PiCCO (P) system were compared to 76 conventionally monitored patients (C). Clinical hemodynamic measurements, organ function (DENVER2 score), and biomarkers were recorded prospectively for the first 20 days after burn injury.
Results
Both cohorts were similar in demographic and injury characteristics. Patients in the PiCCO group received significantly less fluids (p<0.05) with similar urinary output, resulting in a significantly lower positive fluid balance (p<0.05). The central venous pressure (CVP) in the P group was maintained in a more controlled range (p<0.05), associated with a significantly lower heart rate and significantly lower incidence of cardiac and renal failure, p<0.05.
Conclusions
Fluid resuscitation guided by transcardiopulmonary thermo-dilution during hospitalization represents an effective adjunct and is associated with beneficial effects on post-burn morbidity.
Keywords: thermodilution, fluid management, resuscitation, burn injury, pediatric
INTRODUCTION
One of the major determinants for survival of severely burned patients is appropriate fluid resuscitation during the initial phase following injury [1, 2]. It has been shown that unbalanced administration of fluid volume immediately post injury during the acute phase results in significantly higher mortality and complication rates [3]. Over-resuscitation leads to dramatic fluid accumulation with edema formation and complications such as abdominal compartment syndrome or cerebral edema [4]. Under-resuscitation is associated with hemodynamic instability and organ hypoperfusion leading to organ failure and poor clinical outcome [5]. It is therefore imperative to re-evaluate strategies to administer the right amount of fluid to maintain vital organ perfusion without causing fluid overload a massive edema formation [6].
The amount of fluid resuscitation is calculated based on burn size, body weight, body surface, and urinary output, which is often inaccurate and leads to failure in resuscitation volume [7–9]. To improve resuscitation invasive methods to assess the fluid balance such as the measurement of central venous pressure (CVP) and the use of pulmonary artery catheters (PAC) were introduced but these methods are not delivering specific parameters [10, 11]. In the present study, we therefore examined a novel monitoring device that provides more detailed information, the Pulse Contour Cardiac Output (PiCCO) (Pulsion Medical Systems, Munich, Germany). PiCCO uses the technique of thermo dilution according to the Stewart-Hamilton-Method to assess volumetric parameters and allows the intermittent measurement of cardiac output [12]. Additionally, the PiCCO system measures the cardiac index (CI), intrathoracic blood volume index (ITBVI), extra vascular lung water index (EVLWI) and systemic vascular resistance index (SVRI). These parameters give specific results of the fluid demands and fluid shifts and we hypothesized that monitoring and evaluating these data can result in an optimized balance for the patient [12]. The aim of this study was to determine that the use of the PiCCO system and consecutively adjusted fluid management has positive impact on the hospital course, morbidity and mortality in severely burned children.
MATERIALS AND METHODS
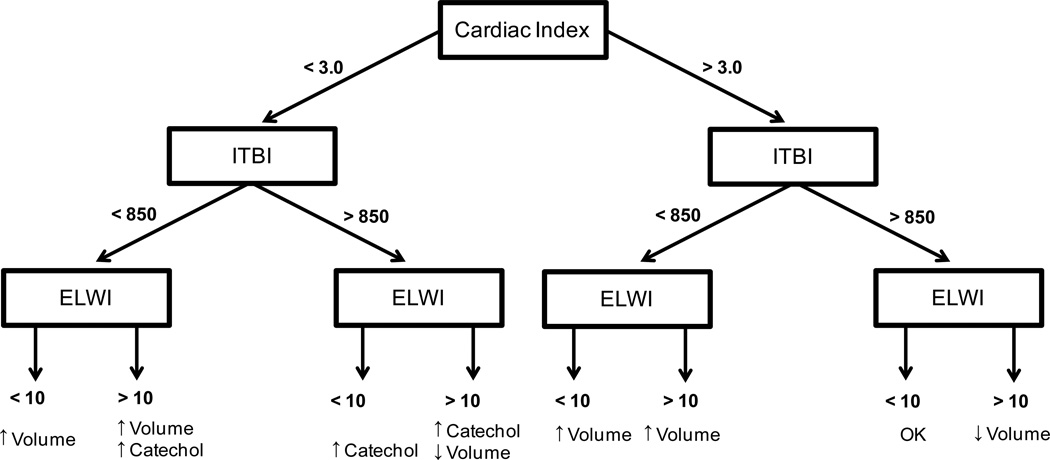
This cohort study analyzed seventy six pediatric patients with severe burns over 30% total burn surface area (TBSA) receiving resuscitation guided by transcardiopulmonary thermo dilution (PiCCO group) monitoring compared to seventy six conventionally (conventional group) resuscitated patients over the first 20 days with similar demographics and injury characteristics. The PiCCO cohort was matched with Control patients for age, gender and injury characteristics. Patients were admitted to the burn unit between 03/1998 and 06/2008 (Control) and 12/2005 and 08/2008 (PiCCO). All patients were resuscitated according to the Galveston formula with 5000 cc/m2 TBSA burned + 2000 cc/m2 TBSA lactated Ringer’s solution given in increments over the first 24 hours. After 24 hours the fluid need was calculated by 3750 ml/m2 BSA burn per day + 1500 ml/m2 BSA per day in the control group. Resuscitation within the PiCCO group was adjusted by the outcomes of the transcardiopulmonary thermo dilution measurements of CI, EVLWI, and ITBVI after the initial 24 hours. Therapeutic targets were to reach normal ranges, especially to maintain EVLWI below 10. Therefore, the PiCCO derived parameters were used to guide treatment decisions of the attending physician on the administration of fluids and inotrope substances according the decision model shown in Figure 1.
Figure 1.
Decision tree for the adjustment of fluid and catecholamine therapy according to PiCCO derived parameters adjusted according the manufacturers recommendations [12].
Within 48 hours of admission, all patients underwent total burn wound excision and the wounds were covered by autograft. Any remaining open areas were covered with homograft. This procedure was repeated until all burn areas were covered with autografts and donor sites were healed.
All patients underwent the same nutritional treatment according to a standardized protocol. The intake was calculated as 1500 kcal/m2 body surface + 1500 kcal/m2 area burn as previously published [13–15]. The nutritional route of choice in our patient population was enteral nutrition via a duodenal (Dobhof) or nasogastric tube. Parenteral nutrition was only given in rare instances if the patient could not tolerate tube feeds.
PiCCO measurements
All patients had a central venous central line and an arterial (mostly femoral artery) access placed upon initial admission. Transpulmonary thermodilution measurements were performed using the Pulsiocath 3 or 4 F thermistor-tipped catheters (Pulsion Medical Systems, Munich, Germany). To determine CI, ITBVI, EVLWI, and SVRI, 10 mL of cooled saline solution (0–6°C) were injected into the cent ral venous circulation using the venous access. Injections were manual and not coordinated with the respiratory cycle. Measurement procedures of each patient were performed at least twice daily. Each procedure consisted of three injections via the venous access and all saline boluses were administered within a maximum time span of 10 minutes. Results were calculated as the mean of these three consecutive measurements. Heart rate, mean arterial pressures, and CVP were calculated from aforementioned variables or recorded directly by the hardware at the same time-points as the thermal bolus injections. Data was recorded and exported to a personal computer with the PICCO-VoLEF-WIN software (version 4.0, Pulsion Medical Systems) combined with the Pulsion PICCOPlus device (PC 8100, software version V6.0, Pulsion Medical Systems).
Patient characteristics/outcome
Patient demographics (age, date of burn and admission, sex, burn size, and depth of burn) and concomitant injuries such as inhalation injury, sepsis, morbidity, and mortality were recorded. Sepsis was defined as a positive blood culture or pathologic tissue identifying the pathogen during hospitalization or at autopsy in combination with at least 3 of the following: leucocytosis or leucopenia (>12,000 or <4,000), hyperthermia or hypothermia (>38.5 or <36.5°C), tachycardia (>150 BPM in childr en), refractory hypotension (systolic BP <90 mmHg), thrombocytopenia (platelets <50,000/mm3), hyperglycemia (serum glucose >240 mg/dl) and enteral feeding intolerance (residuals > 200 cc/hr or diarrhea > 1 L/day) as previously published [13, 14, 16]. We further determined time between operations as a measure for wound healing/re-epithelization.
Resuscitation fluids and urinary output were recorded throughout the study period. Patient data were collected prospectively by physicians, nurses, and research support staff using the clinical information system EMTEK. Data were processed and analyzed with Microsoft Access®, Excel® Microsoft Corporation Inc. (Redmond, WA, USA).
Organ specific Scores
Organ functions of heart, lung, and kidneys were evaluated according the definitions of the DENVER2 score (Tables 1 and 2) [17].
Table 1.
Cardiac Score. Cardiac function was determined by a combination of number and dosage of inotropes administered.
| TnQTable2Patient receives 2 agents: | ||||
|---|---|---|---|---|
| Dose size | (S,S) | (S,M) | (M,M) | (L, anything) |
| Cardiac score | 2 | 2 | 3 | 3 |
| Patient receives 3 or more agents: cardiac score = 3 | ||||
Table 2.
Pulmonary and Renal Score.
| Score | |||||
|---|---|---|---|---|---|
| Component | Measurement | 0 | 1 | 2 | 3 |
| Pulmonary | PaO2/FiO2 | ≥ 250 | 175–249 | 100–174 | < 100 |
| Renal | Creatinine | ≤ 1.8 | (1.8, 2.5) | (2.5, 5.0) | > 5.0 |
Biomarkers
Blood was collected from burn patients at admission and consecutively for 60 days for serum protein and electrolyte analysis. Blood was drawn in a serum-separator collection tube and centrifuged for 10 minutes at 1320 rpm; the serum was removed and stored at −70°C until assayed.
Serum acute phase proteins were determined using HPLC, nephelometry (BNII, Plasma Protein Analyzer Dade Behring, MD), and ELISA techniques.
Ethics and statistics
The study was reviewed and approved by the Institutional Review Board of the University Texas Medical Branch, Galveston, Texas. Prior to the participation, informed consent was obtained from each subject of the study, parent or child’s legal guardian and was documented by signing a written form. Student’s t-test and Chi-square analysis were used where appropriate. Data is expressed as means ± SEM. Statistical significance was accepted at p<0.05.
RESULTS
Demographics
Both study groups were similar in gender, ethnicity, age distribution, injury characteristics regarding severity of burn, and incidence of inhalation injury (Table 3). Initiation of the PiCCO was only in few patients in the first 24 hours therefore the majority of the data is in patients receiving the PiCCO after the initial resuscitation phase.
Table 3.
Patient demographics.
| n | Control 76 |
PICCO 76 |
p-value |
|---|---|---|---|
| Gender | |||
| Male (n) | 49 | 51 | |
| Female (n) | 27 | 25 | |
| Ethnicity | |||
| Afro American (n) | 5 | 7 | |
| Caucasian (n) | 16 | 4 | |
| Hispanic (n) | 53 | 64 | |
| other (n) | 2 | 1 | |
| Age at admit (years) | 7.7 ± 0.6 | 9.1 ± 0.6 | NS |
| Type of burn | |||
| Flame n | 64 (84.2) | 58 (76.3) | NS |
| Scald n | 11 (14.5) | 11 (14.5) | NS |
| Other mechanism n | 1 (1.3) | 7 (9.2) | NS |
| TBSA burn % | 62.2 ± 2.5 | 64.0 ± 2.3 | NS |
| Inhal Injury n (%) | 28 (36.8) | 27 (35.5) | NS |
| TBSA second % | 9.6 ± 13.5 | 14.2 ± 22.5 | NS |
| TBSA third % | 52.2 ± 3.2 | 49.8 ± 3.2 | NS |
| Burn to admit (days) | 2.1 ± 0.2 | 2.0 ± 0.2 | NS |
Both groups are consistent in demographic parameters and injury characteristics.
Clinical outcome
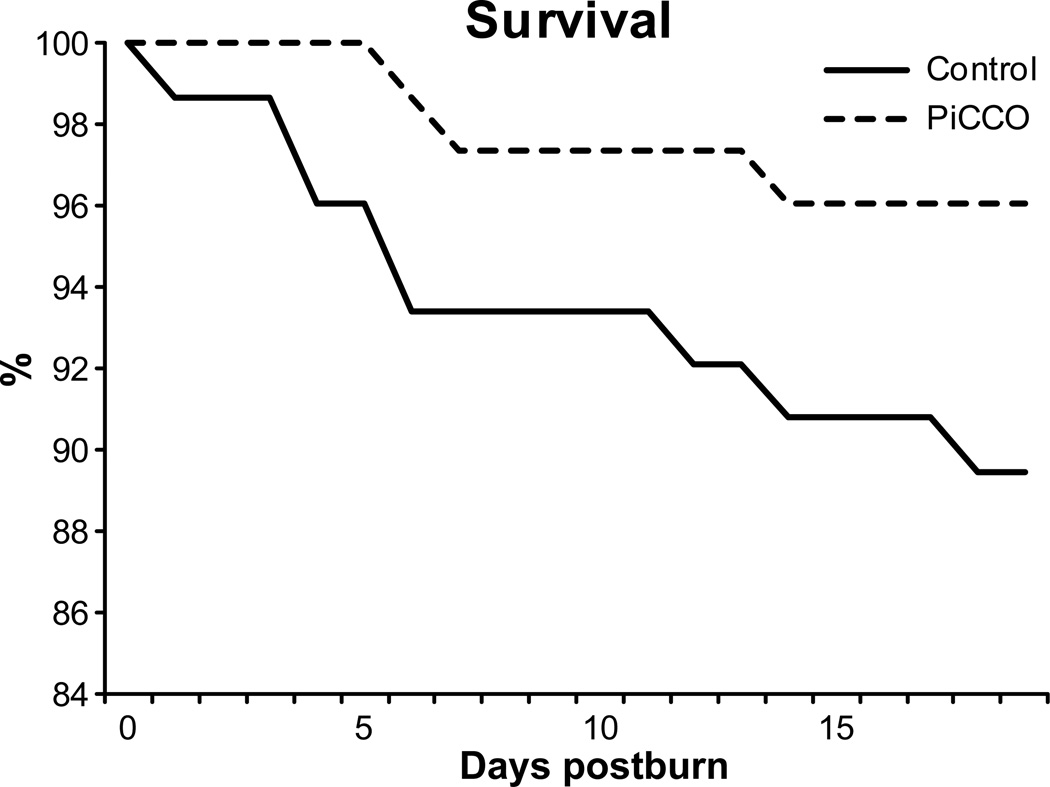
Both patient populations did not show significant differences regarding the clinical outcomes. Required acute surgery, grafting, and treatment duration were not different. The infection rate was slightly higher in the PiCCO group. Interestingly, a not significantly higher incidence of sepsis (p =0.23) and mortality (p=0.22) in the Control group signalized improved outcomes in the PiCCO group (Table 4). However, mortality rates over time visualized by a Kaplan-Meier survival curve reveals an earlier and consistently higher incidence of death in the conventional group during the study period (Fig. 2).
Table 4.
Clinical outcome parameters.
| n | Control 76 |
PICCO 76 |
p-value |
|---|---|---|---|
| Number of OR | 4.8 ± 0.5 | 5.8 ± 0.5 | NS |
| Time btw OR (days) | 5.6 ± 0.2 | 4.8 ± 0.6 | NS |
| LOS ICU (days) | 36.7 ± 4.8 | 34.2 ± 2.9 | NS |
| LOS/TBSA | 0.6 ± 0.1 | 0.6 ± 0.0 | NS |
| Sepsis n (%) | 13 (17.1) | 7 (9.2) | NS |
| Number of Infections | 2.6 ± 0.5 | 3.7 ± 0.5 | NS |
| Died n (%) | 19 (25.0) | 12 (15.8) | NS |
PiCCO and Control patients had a similar rate of clinical interventions with a similar length of stay in the ICU. Patients in the PiCCO group had a slightly higher infection rate but signalized better outcomes with a lower incidence of sepsis and mortality rate (not significant).
Figure 2. Survival curve of the first 20 days of the acute stay.
PiCCO monitored patients showed lower mortality during the crucial initial phase after injury.
Organ specific scores
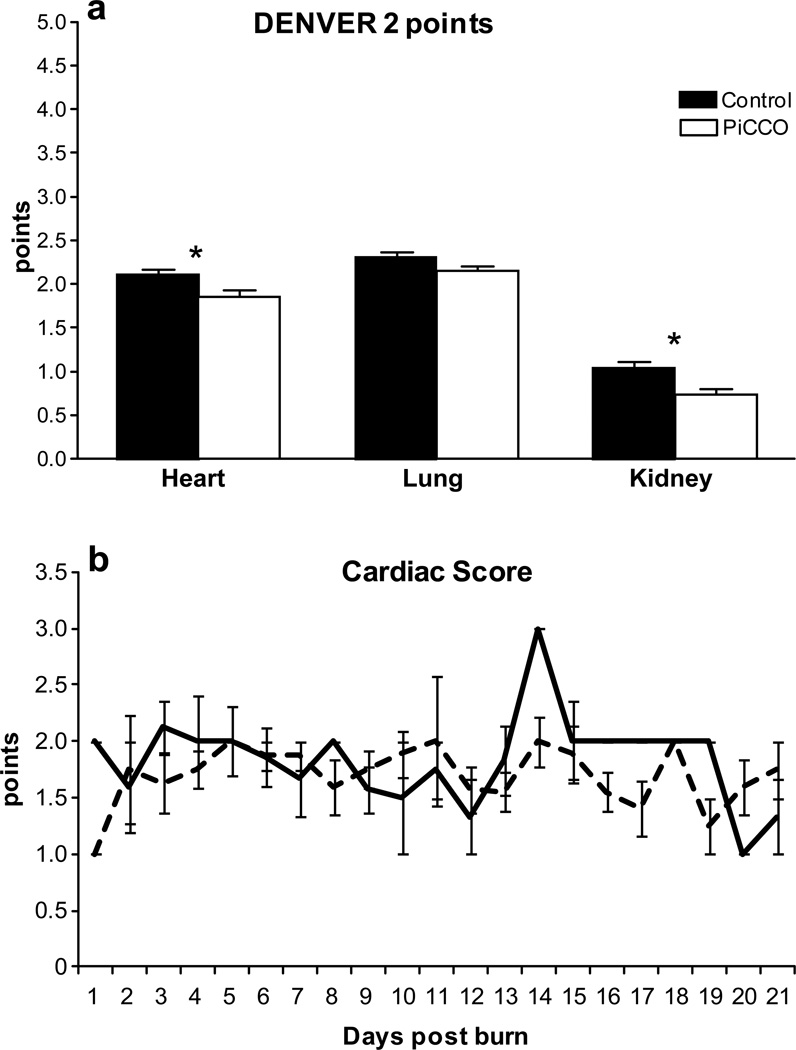
Organ functions of heart, lung, and kidney monitored according the DENVER2 showed significantly (p<0.05) lower cumulative scores in the PiCCO group for cardiac and renal function (Fig. 3) for the first twenty days after burn. The used catecholamines recorded by the cardiac component of the DENVER2 score did not differ significantly between the groups over time (Fig. 3B).
Figure 3. Critical organ functions assessed according the DENVER2 score.
PiCCO monitored patients had less severe cardiac and renal failure compared to the conventional group reflected by the maximum DENVER2 scores for each component (A). Administered catecholamines reflected by the DENVER 2 score for cardiac failure did not differ significantly among the groups over time (B).
Fluid balance and hemodynamic measurements
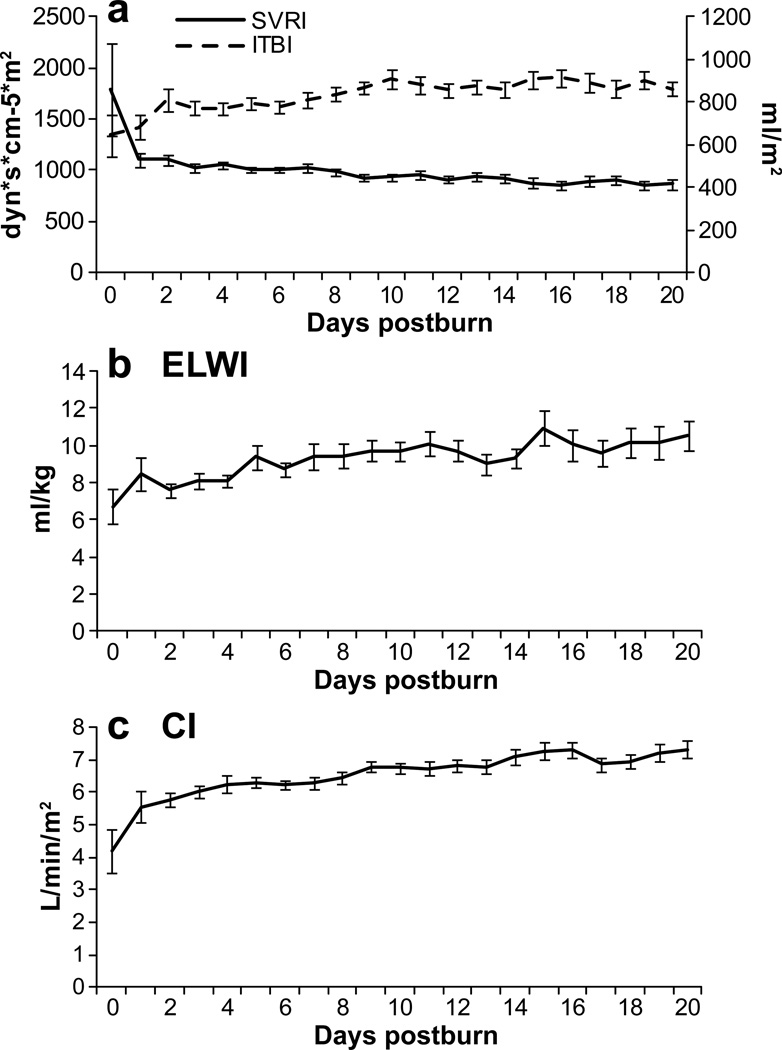
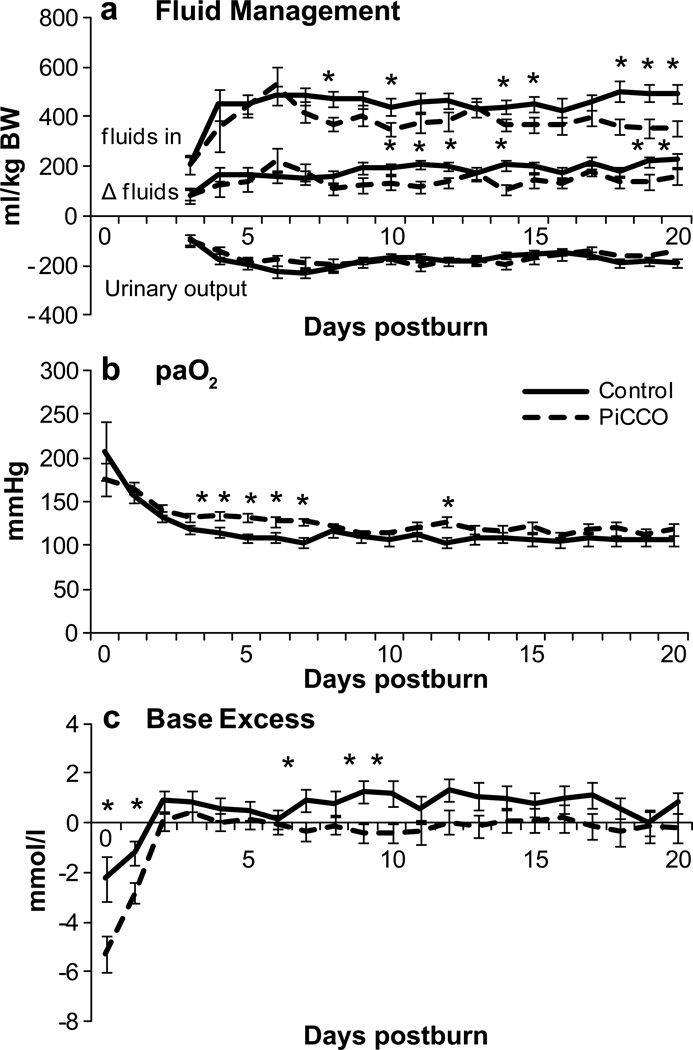
Hemodynamic measurements (Fig. 4) conducted in the PiCCO monitored group showed an increasing extra vascular lung water index starting (ELWI) at 6.7 ml/kg at admit increasing to 10 ml/kg over the first 20 days (normal range 3.0 – 7.0 ml/kg) and CI 4 reaching 10 (normal range 3.0 – 5.0 ml/m2/min). This indicates fluid reposition in lung tissue. The SVRI (normal range 1700 – 2400 dyn*s*cm−5*m2) decreased significantly (p<0.05) after the first day of admit from 1789 dyn*s*cm−5*m2 to consistent levels below 1104 dyn*s*cm−5*m2 for the duration of the observation period. The ITBVI (normal range 850 – 1000 ml/m2) had its lowest measurement at admit with 646 ml/m2, while increasing during the entire study period, and reached normal ranges after day nine.
Figure 4. PiCCO monitored parameters.
Increasing extra vascular lung water index starting (ELWI) indicating for fluid reposition in lung tissue. The systemic vascular resistance index (SVRI) decreased for the duration of the observation period. The intrathoracic blood volume index (ITBVI) reached the normal range after day nine.
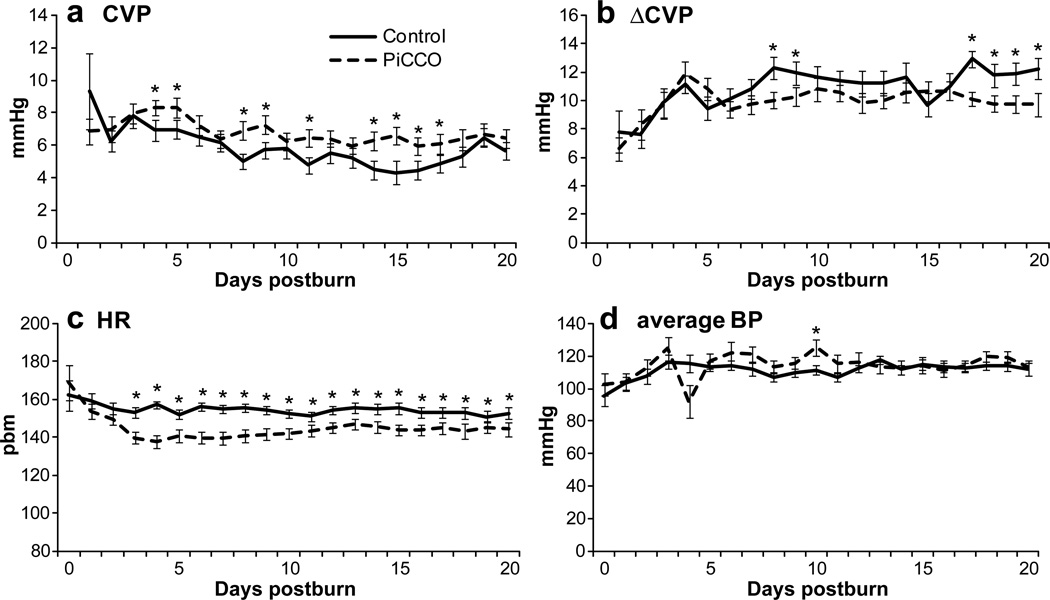
Average daily CVP values of PiCCO-monitored patients were significantly (p<0.05) higher than conventionally monitored patients over the study period (Fig. 5A) and exceed slightly the normal range of 6 mmHg. However, less fluctuation is noted throughout the daily measurements as it can be shown by the analysis of the calculation between the difference in daily minimum and maximum values (Fig. 5B). PiCCO patients showed a lower altitude, partially reaching significance (p<0.05).
Figure 5. Resuscitation outcomes.
PiCCO monitored patients received significantly less fluid with improved diuresis.
These findings of better circulatory values are paralleled by a significantly (p<0.05) lower heart rate (Fig. 5C) combined at similar systemic systolic blood pressure (Fig. 5D) levels in the P group.
Fluid balances recorded as ml/kg showed a significantly higher input of crystalloid and colloid resuscitation solutions in the control group at a similar urinary output (Fig. 6A). Calculated remaining fluid balance was significantly (p<0.05) higher during the first 20 days of hospitalization. This resulted in a higher fluid turnover of the body system. To assure accuracy of the fluid balance, the initial three days after injury were not included in this analysis as the administrations of fluids and urinary output prior to admission were not included in this analysis. Note that this balance does not reflect the intraoperative blood and fluid loss through perspiration.
Figure 6. Hemodynamic measurements.
PiCCO monitoring resulted in less fluctuation in the CVP (A, B) resulting in more stable hemodynamic parameters such as heart rate (C) and systolic blood pressure (D).
Oxygenation of the arterial blood was significantly (p<0.05) improved in the P group (Fig. 6B) combined with a significantly (p<0.05) less fluctuating base excess reflecting the metabolic component of acid-base disorders (Fig. 6C).
Biomarkers
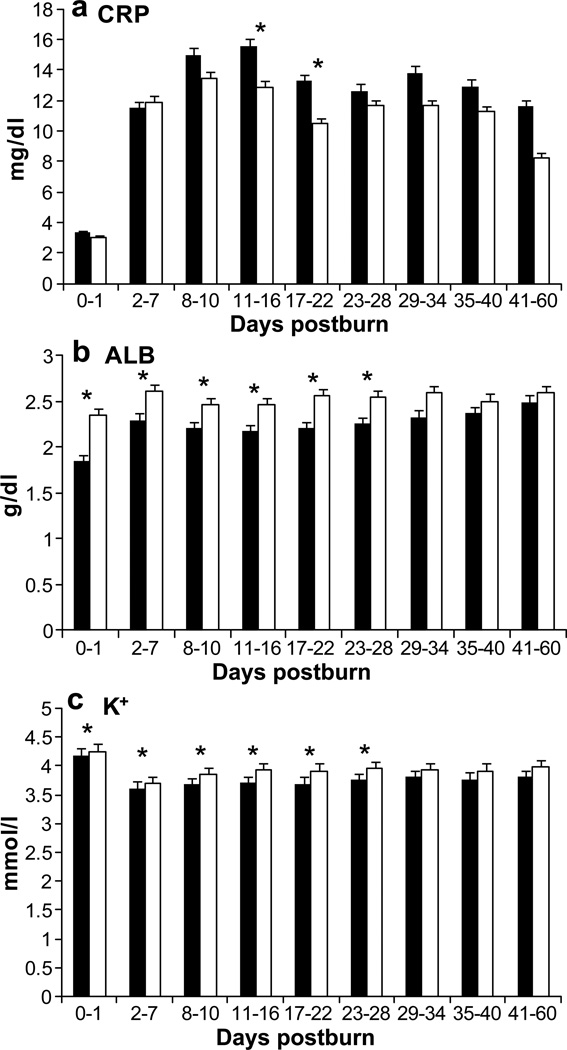
Concomitantly with the observations of organ functions and hemodynamic measurements, C reactive protein (CRP) (Fig. 7A) was significantly (p<0.05) lower in the P group and the plasma levels of albumin and potassium (K) (Fig. 7B, C) were significantly higher (p >0.05) compared to the control group.
Figure 7. Clinical serum parameters.
In PiCCO patients the acute phase protein CRP indicates a lower inflammatory response (A). Albumin (B) and potassium (C) were significantly closer to normal values.
DISCUSSION
The necessity of large amounts of fluid resuscitation immediately after severe burn injury is determined by the intravascular volume loss due to pathological changes in the body of the burn victim [18]. This fluid administration needs very close monitoring not only during the initial phase but also during the critical phase in the intensive care unit (ICU) for stabilization [19]. This clinical treatment phase is characterized in most cases by major operations such as burn wound excisions, grafting, and treatments in the critical care unit maintaining or restoring critical organ functions [20–22]. Therefore, the assessment of the fluid balance is of critical importance to avoid hypo- or hypervolemia which have been shown to be associated with a high complication rate and poor clinical outcome [18]. To evaluate the hydration status, vital parameters such as heart rate, systemic blood, and central venous pressure in addition to various biochemical markers such as albumin, electrolytes, hematocrit, and the urinary output are used [7, 23, 24]. However, in order to refine the volume substitution therapy, additional information on specific parameters for volume distribution would be advantageous as an assurance for optimized organ perfusion and to prevent the development of edemas.
In this present study, we were able to show that adjusted fluid management following monitoring by the PiCCO system has beneficial effects on the hospital course and patient outcomes. Conventionally (Control group) monitored patients showed a remarkable higher incidence of sepsis and mortality at a similar infection rate. As sepsis was defined by the ACCP/SCCM criteria adjusted for burns [25, 26], we attribute the lower sepsis rate in the PiCCO group to improved hemodynamic parameters which are part of the score assessment. The relatively small number of patients enrolled in this study must be taken into account in regards to the remarkable but not statistically significant difference between both study groups in terms of sepsis and mortality.
Relevant organ functions of the heart and kidneys showed a significant improvement according to the PiCCO measurement-treated group [27]. We attribute these results to better maintenance of systemic blood pressures leading to fewer requirements of catecholamines and improved perfusion of the kidneys which lowers systemic creatinin. The results of the fluid distribution measured in the PiCCO group by SVRI and ITBI correlate to this observation of enhanced central perfusion. Although it can be observed that the systemic vascular resistance after admission dropped tremendously after the first two days of admission, the intrathoracic blood volume remained at relatively constant levels and returned to normal levels after the first week. The fall of SVRI during the initial phase can be partially attributed to the deep sedation of the patients and is in accordance with other studies which attributed this change to oxidative stress and due to inflammatory processes and SIRS [28, 29]. However, we show that the maintenance of the central perfusion was stable, a crucial importance for critical organ functions. Moreover, the extravascular lung water index, one of the critical outcome parameters, did not rise significantly over the observed study period. Because this particular data set cannot be derived for the conventionally treated group, we show that commonly used parameters were improved in the PiCCO group. The daily average CVP was consistently higher and showed less fluctuation in the daily measurements as observed by the lower altitude of the differences between the minimum and maximum values in the individual patient. This indicates that the fluid intake is sustained more constantly throughout the day, thereby significantly balancing hydration. We assume that CVP values slightly above normal may compensate for the loss of peripheral vascular resistance and does not affect the hospital course and outcomes in a negative way. Similar observations can be made regarding the fluid intake and urinary output. Conventionally treated patients were resuscitated with significantly more fluids, but did not produce more urine over the study period. This resulted in significantly higher positive fluid balance throughout the acute hospital stay. Assuming that both patient populations received similar treatment regarding the replacement of plasma proteins and electrolytes, this correlates with significantly higher levels of albumin and potassium.
Other important and routinely used parameters for the assessment of the hydration status such as the systemic blood pressure and the heart rate also showed significant improvement in the PiCCO group. Higher systemic systolic blood pressure levels indicate better systemic perfusion and significantly lower heart rate which designates improved cardiac output combined with less cardiac work. These results, combined with the results of heart rate and blood pressure, provide a better shock index (data not shown) within the PiCCO group. This index has been shown to reflect reliable the hemodynamic state of the intensive care patient and a low index are associated with a higher mortality [30, 31]. This value expresses the direct cardiac work compared to perfusion outcomes. Especially in burn patients, a lower heart rate is targeted as it has been shown that due to stressors increased cardiac work correlates with morbidity and mortality and therefore needs to be treated [32]. These improved two major circulatory parameters followed by a significantly better oxygenation of the arterial blood, peripheral circulation and metabolism reflected by a less great base excess in the PiCCO group.
This study has several limitations that need to be addressed. This is not a prospective randomized trial and hence the data need to be interpreted cautiously. Despite better fluid management during hospital course, the PiCCO was not used where one would expect that its use would have the greatest benefit, during the crucial and important first 24 hours post-burn. As mentioned before, the majority of our patients were admitted after 24 hours, and therefore, we have only little data on the effect of the PiCCO during the initial phase. Based on our data indicating that the PiCCO optimized fluid management during the later phase, we hypothesize that PiCCO use may have also benefits in the initial resuscitation phase. This should be studied in a prospective randomized fashion. Another limitation is that this study was conducted in children and therefore the benefit of the PiCCO system in burned adults or elderly has to be shown. Lastly, the efficacy of the PiCCO system depends on the experience of the burn team. There is clearly a learning curve for the burn team using the system. Analysis and interpretation of the PiCCO depend on the accuracy of the test and therefore the calibration and testing have to be conducted with great care. In summary, this trial indicates that refined fluid resuscitation through utilization of supplementary parameters which affects direct outcome such as the ELWI, SVRI, and ITBI, enhancing the fluid resuscitation and presents a tool in improving these outcomes. We were able to show that major outcome parameters such as heart rate, systemic blood pressure, oxygenation, and for peripheral metabolism are improved by fluid guided therapy. The next step need to be a prospective randomized controlled trial comparing the PiCCO adjusted fluid algorithms to standard fluid algorithms including the first 24 hours post-burn in severely burned children, adults and elderly.
ACKNOWLEDGMENTS
This study was supported by grants from the American Surgical Association Foundation, Shriners Hospitals for Children (84080, 8660, 8760, and 9145), National Institutes of Health (R01-GM56687, T32 GM008256, and P50 GM60338), and NIDRR (H133A020102, H133A070026 and H133A70019).CCF is an ITS Career Development Scholar supported, in part, by NIH KL2RR029875 and NIH UL1RR029876.
We thank all the individuals who participated in this clinical trial. We also would like to thank the research staff for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no competing interests.
REFERENCES
- 1.Klein MB, Hayden D, Elson C, et al. The association between fluid administration and outcome following major burn: a multicenter study. Ann Surg. 2007;245:622. doi: 10.1097/01.sla.0000252572.50684.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santry HP, Alam HB. Fluid resuscitation: past, present, and the future. Shock. 2010;33:229. doi: 10.1097/SHK.0b013e3181c30f0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoskins SL, Elgjo GI, Lu J, et al. Closed-loop resuscitation of burn shock. J Burn Care Res. 2006;27:377. doi: 10.1097/01.BCR.0000216512.30415.78. [DOI] [PubMed] [Google Scholar]
- 4.Gore DC, Hawkins HK, Chinkes DL, et al. Assessment of adverse events in the demise of pediatric burn patients. J Trauma. 2007;63:814. doi: 10.1097/TA.0b013e31811f3574. [DOI] [PubMed] [Google Scholar]
- 5.Brusselaers N, Monstrey S, Colpaert K, et al. Outcome of acute kidney injury in severe burns: a systematic review and meta-analysis. Intensive Care Med. 2010;36:915. doi: 10.1007/s00134-010-1861-1. [DOI] [PubMed] [Google Scholar]
- 6.Shah A, Connolly CM, Kirschner RA, et al. Evaluation of hyperdynamic resuscitation in 60% TBSA burn-injured sheep. Shock. 2004;21:86. doi: 10.1097/01.shk.0000101666.49265.b4. [DOI] [PubMed] [Google Scholar]
- 7.Warden GD. Fluid resuscitation and early management. In: Herndon DN, editor. Total Burn Care. Philadelphia: Saunders Elsevier; 2007. p. 107. [Google Scholar]
- 8.Blumetti J, Hunt JL, Arnoldo BD, et al. The Parkland formula under fire: is the criticism justified? J Burn Care Res. 2008;29:180. doi: 10.1097/BCR.0b013e31815f5a62. [DOI] [PubMed] [Google Scholar]
- 9.Brazeal BA, Honeycutt D, Traber LD, et al. Pentafraction for superior resuscitation of the ovine thermal burn. Crit Care Med. 1995;23:332. doi: 10.1097/00003246-199502000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993–2004. JAMA. 2007;298:423. doi: 10.1001/jama.298.4.423. [DOI] [PubMed] [Google Scholar]
- 11.McKinley BA, Sucher JF, Todd SR, et al. Central venous pressure versus pulmonary artery catheter-directed shock resuscitation. Shock. 2009;32:463. doi: 10.1097/SHK.0b013e3181a20ba9. [DOI] [PubMed] [Google Scholar]
- 12.Pulsion PiCCO Technology. Munich, Germany: Pulsion Medical Systems AG; 2006. [Google Scholar]
- 13.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 15.Mlcak RP, Jeschke MG, Barrow RE, et al. The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg. 2006;244:121. doi: 10.1097/01.sla.0000217678.78472.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeschke MG, Boehning DF, Finnerty CC, et al. Effect of insulin on the inflammatory and acute phase response after burn injury. Crit Care Med. 2007;35:S519. doi: 10.1097/01.CCM.0000282027.10288.10. [DOI] [PubMed] [Google Scholar]
- 17.Moore FA, Sauaia A, Moore EE, et al. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Barrow RE, Jeschke MG, Herndon DN. Early fluid resuscitation improves outcomes in severely burned children. Resuscitation. 2000;45:91. doi: 10.1016/s0300-9572(00)00175-1. [DOI] [PubMed] [Google Scholar]
- 19.Salinas J, Drew G, Gallagher J, et al. Closed-loop and decision-assist resuscitation of burn patients. J Trauma. 2008;64:S321. doi: 10.1097/TA.0b013e31816bf4f7. [DOI] [PubMed] [Google Scholar]
- 20.Barret JP, Herndon DN. Modulation of inflammatory and catabolic responses in severely burned children by early burn wound excision in the first 24 hours. Arch Surg. 2003;138:127. doi: 10.1001/archsurg.138.2.127. [DOI] [PubMed] [Google Scholar]
- 21.Chrysopoulo MT, Jeschke MG, Dziewulski P, et al. Acute renal dysfunction in severely burned adults. J Trauma. 1999;46:141. doi: 10.1097/00005373-199901000-00024. [DOI] [PubMed] [Google Scholar]
- 22.McGwin G, Jr, George RL, Cross JM, et al. Gender differences in mortality following burn injury. Shock. 2002;18:311. doi: 10.1097/00024382-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Guha SC, Kinsky MP, Button B, et al. Burn resuscitation: crystalloid versus colloid versus hypertonic saline hyperoncotic colloid in sheep. Crit Care Med. 1996;24:1849. doi: 10.1097/00003246-199611000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Tokyay R, Zeigler ST, Heggers JP, et al. Effects of anesthesia, surgery, fluid resuscitation, and endotoxin administration on postburn bacterial translocation. J Trauma. 1991;31:1376. doi: 10.1097/00005373-199110000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 26.Silver GM, Klein MB, Herndon DN, et al. Standard operating procedures for the clinical management of patients enrolled in a prospective study of Inflammation and the Host Response to Thermal Injury. J Burn Care Res. 2007;28:222. doi: 10.1097/BCR.0B013E318031AA44. [DOI] [PubMed] [Google Scholar]
- 27.Xiao N, Wang XC, Diao YF, et al. Effect of initial fluid resuscitation on subsequent treatment in uncontrolled hemorrhagic shock in rats. Shock. 2004;21:276. doi: 10.1097/01.shk.0000110622.42625.cb. [DOI] [PubMed] [Google Scholar]
- 28.Foldi V, Csontos C, Bogar L, et al. Effects of fluid resuscitation methods on burn trauma-induced oxidative stress. J Burn Care Res. 2009;30:957. doi: 10.1097/BCR.0b013e3181bfb75e. [DOI] [PubMed] [Google Scholar]
- 29.Demling RH. The burn edema process: current concepts. J Burn Care Rehabil. 2005;26:207. [PubMed] [Google Scholar]
- 30.Rady MY, Smithline HA, Blake H, et al. A comparison of the shock index and conventional vital signs to identify acute, critical illness in the emergency department. Ann Emerg Med. 1994;24:685. doi: 10.1016/s0196-0644(94)70279-9. [DOI] [PubMed] [Google Scholar]
- 31.Kreit JW. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest. 2004;125:1539. doi: 10.1378/chest.125.4.1539. [DOI] [PubMed] [Google Scholar]
- 32.Baron PW, Barrow RE, Pierre EJ, et al. Prolonged use of propranolol safely decreases cardiac work in burned children. J Burn Care Rehabil. 1997;18:223. doi: 10.1097/00004630-199705000-00008. [DOI] [PubMed] [Google Scholar]