Abstract
Syntheses of the C(15)–C(27) fragments of chaxamycins A/D, rifamycin S, and the C(12)–C(24) fragment of salinisporamycin have been accomplished in ten steps from commercially available starting materials. Three crotylboron reagents were utilized to construct the seven contiguous stereocenters in these fragments with excellent stereoselectivity.
Aldehyde allylboration, synthesis of propionate fragments of chaxamycins A and D, rifamycin S and salinisporamycin
Introduction
Ansamycin antibiotics such as the rifamycins1 are macrolides consisting of an aromatic chromophore bridged by an aliphatic chain linking two nonadjacent positions on the aromatic moiety. In addition to their antimicrobial activities against many Gram positive and some Gram-negative bacteria, many ansamycins also exhibit antiviral activities.1 Furthermore, it is well documented that some members of the ansamycin family selectively interact with the ATP-binding pocket in the N-terminal domain of the heat shock protein 90 (Hsp90) and demonstrate antitumor activity.2
Because of the structural complexities coupled with diverse biological activities, extensive efforts have been devoted to the syntheses of ansamycin natural products. In 1980, Kishi and coworkers reported the first total synthesis of rifamycin S.3 Following his landmark work, many strategies have been developed for the synthesis of the polyketide ansa chain of rifamycin S.4
Recently, salinisporamycin5,6 and several new members of the ansamycin antibiotic family, chaxamycins A–D,7 were reported (Figure 1). Salinisporamycin was isolated in 2009 from Salinispora arenicola. Subsequent biological studies revealed that salinisporamycin not only showed antimicrobial activity, but also inhibited the growth of A549 cells (the human lung adenocarcinoma cell line) with an IC50 value of 3 μg/mL.5 Chaxamycins A–D were isolated from Streptomyces sp. strain C34 obtained from soil collected in the hyper-arid Chilean Atacama Desert.7 Among the chaxamycins, chaxamycin D displayed a highly selective antibacterial activity against S. aureus ATCC 25923 with an IC50 value of 0.05 μg/mL.
Figure 1.

Structures of chaxamycins A and D, salinisporamycin and rifamycin S
As illustrated in Figure 1, chaxamycins A and D have ansa chains that are very similar to the analogous fragments of salinisporamycin and rifamycin S (shown in red in Figure 1). One intriguing structural feature of these natural products is the anti,anti-stereotriad embedded in the polyketide chain (highlighted in yellow in Figure 1). In order to access this requisite anti,anti-stereotriad,8 many creative approaches, including epoxide ring opening reactions,3,4k the desymmetrization of meso intermediates4d,4l,4m or elaboration of intermediates derived from hetero-Diels-Alder reactions,4f have been developed.
In 1987, our laboratory reported a stereoselective synthesis of the ansa chain of rifamycin S4g,4j using the tartrate-based crotylboronate reagents.9 In initial studies, we attempted a mismatched double asymmetric crotylboration10 of the β-branched chiral aldehyde 1 using our tartrate ester modified crotylboronate reagent for the synthesis of the anti,anti-stereotriad A. However, the tartrate-based crotylboronate reagent failed to override the intrinsic diastereofacial selectivity of the chiral aldehyde, and a mixture of diastereomers was obtained with the desired anti,anti-stereotriad A only as a minor product. Therefore, we pursued an alternative strategy in which the anti,anti-stereotriad 2, which can be prepared with good selectivity,4g was used at the starting point for the synthesis (Figure 2a).
Figure 2.

Strategy for synthesis of the anti,anti-stereotriad 7
Recently, we described highly diastereoselective syntheses of anti,anti-stereotriads, such as 5, using mismatched double asymmetric δ-stannylcrotylboration reactions11 of chiral aldehydes with the chiral crotylborane reagents (R)-(E)-4 or (S)-(E)-412 (Figure 2b). Because of the exceptional enantiofacial selectivity of reagents (S)-(E)-4 and (R)-(E)-4, we were intrigued whether the anti,anti-stereotriad within the polyketide chain segment of the structures in Figure 1 could be prepared using reagent (R)-(E)-4 (Figure 2c). This would enable these syntheses to be accomplished in a more direct, step-wise efficient manner without resorting to the pseudo two-directional chain synthesis approach in our first generation synthesis of the rifamycin S ansa chain.4j The results of this study are reported in this manuscript.
Results and Discussion
Our retrosynthetic analysis for synthesis of the polypropionate units 8 and 9 is summarized in Figure 3. We envisioned that 8 and 9 could be assembled from aldehyde 10, crotylborane (S)-(E)-412 and vinyl iodides 11.6a,13 To access the anti,anti-stereotriad embedded in aldehyde 10, we planned to utilize the mismatched double asymmetric δ-stannylcrotylboration of aldehyde 13 with the chiral crotylborane reagent (R)-(E)-4.11,12
Figure 3.

Retrosynthetic analysis of stereoheptads 8 and 9
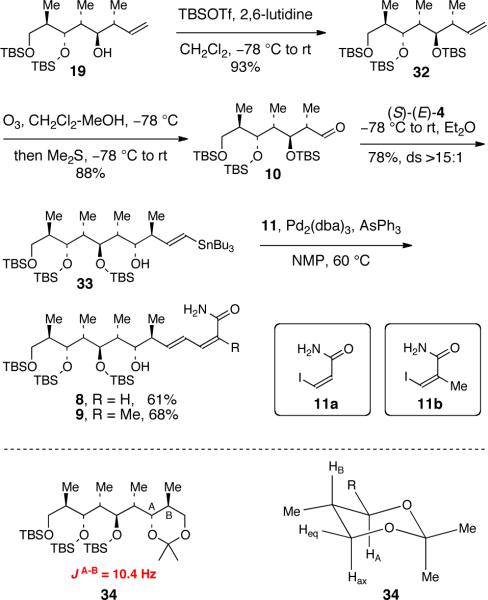
The synthesis of aldehyde 13 is summarized in Scheme 1. Commercially available ester 14 was converted into aldehyde 15 in two steps according to known procedures.14 Crotylboration of 15 using Brown's (Z)-crotylborane reagent 16 gave homoallylic alcohol 17 in 81% yield.15 Protection of the secondary alcohol of 17 (TBSOTf, 2,6-lutidine) gave the known TBS ether 18 in near quantitative yield.16 Subsequent ozonolysis reaction of 18 provided aldehyde 13 in 91% yield.
Scheme 1.

Synthesis of Aldehyde 13
Addition of aldehyde 13 to a solution of (R)-(E)-4 (generated as described previously11,12) at −78 °C followed by warming the reaction mixture to ambient temperature provided the targeted anti,anti-stereotriad 12 in 71% yield and with >15:1 diastereoselectivity (Scheme 2a). Subsequent protodestannylation of 12 under acidic conditions (TsOH·H2O)12b provided alcohol 19 in 92% yield.
Scheme 2.
Crotylboration Reactions of Aldehyde 13
The intrinsic diastereofacial preference of aldehyde 13 was assessed by using the crotylboration reaction with the achiral pinacol (E)-crotylboronate 20 (Scheme 2b). Interestingly, formation of the major isomer 3,4-anti-4,5-syn-stereotriad 21 was only slightly favored (~1.4:1) over the anti,anti-stereotriad 19. The stereochemistry of the major product 21 was assigned following ozonolysis with reductive workup using NaBH4 followed by conversion of the 1,3-diol to the corresponding acetonide, the 2,3-stereochemistry of which was easily assigned by 1H NMR analysis as summarized in the Supporting Information. The ratio of 21 and 19 suggests that the two competing transition states, TS-1 and TS-2, which lead to the formation of 21 and 19, respectively, must be relatively close in energy.
As shown in Scheme 2b, the slightly favored transition state TS-1 proceeds under substrate/Felkin-Anh17 control to give the 3,4-anti-4,5-syn-stereotriad 21. A relatively minor gauche-pentane interaction18 between the methyl group of the crotyl unit and the methyl group of aldehyde 13 occurs in TS-1 (shown in red). On the other hand, the competing anti-Felkin-Anh transition state TS-2 gives anti,anti-stereotriad 19. Gauche-pentane interactions18 between the methyl group of the crotylboronate reagent and the side chain of aldehyde 13 (indicated in the red box in TS-2, Scheme 2b) should develop in TS-2. Close examination of TS-2 reveals that the side chain of aldehyde 13 can adopt a conformation such that the secondary OTBS group is positioned away from the methyl group of the crotylboronate and only a hydrogen atom is within close proximity to the methyl group of the crotyl unit (shown in red in TS-2). Consequently, it appears that the Felkin-Anh TS-1 and the anti-Felkin-Anh TS-2 have comparable non-bonded steric interactions between aldehyde 13 and the achiral pinacol crotylboronate 20. This analysis is consistent with experimental results that the two competing transition states for the crotylboration reaction are close in energy.
It is well documented that many classical chiral crotylating reagents have demonstrated ability to overcome modest intrinsic diastereofacial selectivities (e.g., < 3–4:1 ds) of chiral aldehydes.4j,19 Indeed, when aldehyde 13 was treated with the tartrate derived chiral crotylboronate reagent 22,9 the anti,anti-stereotriad 19 was obtained in 72% yield and with 13:1 diastereoselectivity (Scheme 2c).
It is appreciated that the intrinsic diastereofacial selectivity preferences of chiral aldehydes in reactions with achiral crotylboron reagents (as well as with other crotylmetal reagents) is dependent on the stereochemistry of the chiral aldehyde substrate as well as the protecting groups included in the substrates (e.g., compare results of mismatched double asymmetric reactions of 1 and 13 with the tartrate ester crotylboronate, 22, presented above).19d Indeed, as summarized in Scheme 3, the crotylboration reactions of aldehydes 23, 24, and 25, which are diastereomers of 13, with achiral pinacol crotylboronate (20) provide product mixtures ranging from 3:1 (for 23) to > 100:1 (for 25). The β-alcohol protecting groups of aldehydes 24 and 25 project into space occupied by the (E)-methyl group of the crotylborane reagent in transition states TS-4 and TS-5 (which lead to the minor anti, anti stereotriads 29 and 31, respectively), thereby destabilizing these transition states and making it difficult to access structures like 29 and 31.20 Therefore, while conventional chiral crotylmetal reagents may be capable of overriding the intrinsic diastereofacial preference of substrates such as 13 and 23 in mismatched double asymmetric reactions,19 a much more highly enantioselective reagent such as 4 is required to achieve acceptable stereochemical control in mismatched double asymmetric reactions with chiral aldehydes with intrinsic diastereofacial selectivities >5:1 (and especially >10:1).11
Scheme 3.
Intrinsic Diastereofacial Selectivity of Chiral Aldehydes Related to 13 is Dependent on Stereochemistry of the Aldehyde Substrate
Completion of the syntheses of 8 and 9 is summarized in Scheme 4. The secondary alcohol of stereopentad 19 was protected as a TBS ether under standard conditions (TBSOTf, 2,6-lutidine). Ozonolysis of TBS ether 32 gave aldehyde 10 in 88% yield. Matched double asymmetric crotylboration of 10 with (S)-(E)-4 provided δ-stannyl-homoallylic alcohol 33 in 78% yield and with >15:1 diastereoselectivity. Other diastereomers were not detected in the reaction mixture. The anti-relative stereochemistry of two newly formed stereocenters in 33 was supported by the coupling constant analysis of the acetonide derivative 34 that was prepared from 33 by protiodestannylation, ozonolysis, reductive workup of the ozonoide using NaBH4, and then acetonide formation (please see SI for details). Finally, a Pd-catalyzed Stille coupling21 of vinyl stannane 33 with vinyl iodide 11a13 provided 8 in 61% yield. Similarly, coupling of vinyl stannane 33 with vinyl iodide 11b6a afforded 9 in 68% yield under analogous reaction conditions.
Scheme 4.
Completion of the Syntheses of 8 and 9
Conclusion
Syntheses of the polypropionate fragments of chaxamycins A/D, salinisporamycin and rifamycin S were accomplished in ten steps from commercially available starting materials. Three crotylboron reagents were utilized to construct the seven contiguous stereocenters in these polypropionate units. The vinylstannane unit of alcohol 33 derived from the matched double asymmetric crotylboration of aldehyde 10 with crotylborane (S)-(E)-4 allowed direct C–C bond formation using a subsequent Stille coupling reaction.21 Other synthetic applications of crotylborane reagents (S)-(E)-4 and (R)-(E)-4 will be reported in due course.
Experimental Section22
(3R,4R,5R,6R,7R)-6,8-bis((tert-butyldimethylsilyl)oxy)-3,5,7-trimethyloct-1-en-4-ol (19)
A 50-mL, pear-shaped flask equipped with a rubber septum and argon inlet needle was charged with 4Å molecular sieves (freshly activated under vacuum, 200 mg) and anhydrous toluene (15 mL). To this flask was added a solution of aldehyde 13 (750 mg, 2.0 mmol, prepared in five steps from commercially available ester 14 according to known precedures14–16) in anhydrous toluene (5 mL). The mixture was then cooled to −78 °C and a solution of (E)-crotylboronate 229 in toluene (3.8 mL, ~0.8 M, 3.0 mmol, 1.5 equiv) was added. The mixture was kept at −78 °C and stirred for 24 h. The reaction was quenched by addition of aqueous 3 N NaOH (5 mL) and stirred vigorously for 2 h. Brine (5 mL) was added, the organic layer was separated and the aqueous layer was extracted with Et2O (3 × 5 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. Purification of the crude product was performed by flash column chromatography (gradient elution, hexane-Et2O = 100:1 to 10:1), which provided anti, anti-stereotriad 19 (620 mg, 72% yield; ds = 13:1) as a colorless oil: [α]D 28.5 = +15.8° (c 0.75, CHCl3); 1H NMR (400 MHz, CDCl3) δ 5.88 (ddd, J = 16.8, 10.4, 8.4 Hz, 1H), 5.02–5.08 (m, 2H), 3.85 (dd, J = 6.4, 2.0 Hz, 1H), 3.74 (dd, J = 10.0, 4.8 Hz, 1H), 3.50 (ddd, J = 10.4, 4.0, 2.4 Hz, 1H), 3.45 (dd, J = 10.0, 7.2 Hz, 1H), 3.09 (bs, 1H), 2.29–2.37 (m, 1H), 1.88–1.98 (m, 1H), 1.71–1.79 (m, 1H), 1.12 (d, J = 6.8 Hz, 3H), 0.94 (d, J = 6.8 Hz, 3H), 0.90 (s, 18H), 0.82 (d, J = 7.2 Hz, 3H), 0.09 (s, 3H), 0.07 (s, 3H), 0.04 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 139.4, 115.7, 76.8, 76.7, 66.1, 40.9, 40.8, 39.7, 26.33, 26.32, 18.7, 18.5, 18.4, 15.4, 13.0, −3.9, −4.2, −4.9, −5.0; IR (neat) 3501, 2959, 2932, 2887, 2859, 1472, 1464, 1390, 1362, 1257, 1084, 1024, 1006, 913, 837, 776, 672 cm−1; HRMS (ESI) m/z for C23H51O3Si2 [M+H]+ calcd 431.3377, found 431.3371.
(2Z,4E,6S,7S,8R,9R,10S,11R,12R)-9,11,13-tris((tert-butyldimethylsilyl)oxy)-7-hydroxy-6,8,10,12-tetramethyltrideca-2,4-dienamide (8)
A 50-mL, pear-shaped flask equipped with a rubber septum and argon inlet needle was charged with alcohol 19 (600 mg, 1.4 mmol) and anhydrous CH2Cl2 (15 mL). The solution was cooled to −78 °C and 2,6-lutidine (300 mg, 2.8 mmol) was added. After 10 min, tert-butyldimethylsilyl trifluoromethanesulfonate (555 mg, 2.1 mmol) was added at −78 °C. The reaction mixture was allowed to warm to ambient temperature and stirred for 8 h. The reaction was quenched with a saturated solution of NaHCO3 (5 mL) and stirred for 1 h. Brine (5 mL) was added, the organic layer was separated and the aqueous layer was extracted with CH2Cl2 (3 × 5 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. Purification of the crude product was performed by flash column chromatography (gradient elution, hexane:Et2O = 500:1 to 50:1), which provided TBS ether 32 (708 mg, 93% yield) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 5.98 (ddd, J = 18.0, 10.8, 8.0 Hz, 1H), 4.93–4.99 (m, 2H), 3.76 (dd, J = 4.0, 4.0 Hz, 1H), 3.67 (dd, J = 10.0, 5.2 Hz, 1H), 3.57 (dd, J = 6.8, 2.4 Hz, 1H), 3.39 (dd, J = 10.0, 8.0 Hz, 1H), 2.38–2.45 (m, 1H), 1.73–1.82 (m, 2H), 1.04 (d, J = 6.8 Hz, 3H), 0.91 (s, 9H), 0.90 (d, J = 6.8 Hz, 3H), 0.89 (s, 18H), 0.81 (d, J = 7.2 Hz, 3H), 0.10 (s, 3H), 0.08 (s, 3H), 0.07 (s, 3H), 0.06 (s, 3H), 0.043 (s, 3H), 0.039 (s, 3H).
A stream of ozone in O2 was bubbled through a solution (initially light red, with Sudan III as the indicator) of TBS ether 32 (270 mg, 0.5 mmol) in dichloromethane (4 mL) and MeOH (1 mL) at −78 °C until the light red solution became colorless. The solution was sparged with nitrogen to remove any excess ozone, then dimethylsulfide (5 mL) was added at –78 °C. The reaction mixture was allowed to warm to ambient temperature and stirred for 24 h. The reaction mixture was filtrated through a pad of Celite. The solution was concentrated under reduced pressure. Purification of the crude product was performed by flash column chromatography (gradient elution, hexane:Et2O = 100:1 to 10:1), which provided aldehyde 10 (240 mg, 88% yield) as a colorless oil. . 1H NMR (400 MHz, CDCl3) δ 9.82 (d, J = 1.6 Hz, 1H), 3.90 (dd, J = 6.4, 2.0 Hz, 1H), 3.81 (dd, J = 4.0, 3.2 Hz, 1H), 3.57 (dd, J = 10.4, 6.8 Hz, 1H), 3.39 (dd, J = 10.0, 6.8 Hz, 1H), 2.59 (qdd, J = 7.2, 1.6, 1.6 Hz, 1H), 1.79–1.93 (m, 2H), 1.13 (d, J = 6.8 Hz, 3H), 0.90 (s, 9H), 0.894 (s, 9H), 0.89 (s, 9H), 0.87 (d, J = 7.2 Hz, 3H), 0.84 (d, J = 6.8 Hz, 3H), 0.10 (s, 3H), 0.08 (s, 3H), 0.07 (s, 3H), 0.06 (s, 3H), 0.05 (s, 3H), 0.04 (s, 3H).
Crystalline (dIpc)2BH (286 mg, 1.0 mmol) was weighed into a round bottom flask containing a stir bar in a glove box. (Note: The crystalline borane should be crushed and pulverized to fine powder with a glass rod in order to ensure efficient allene hydroboration). The flask was capped with a rubber septum and removed from the glove box and placed in a cold bath (0 °C). Ether (1.5 mL) was added slowly to the flask and the mixture (suspension) was cooled to 0 °C. Racemic 1-stannyl-1,2-butadiene (344 mg, 1.0 mmol) was added neat via a microliter syringe. This mixture was stirred for 5 h at 0 °C, during which time the solid (dIpc)2BH dissolved to leave a colorless solution. The reaction mixture was cooled to −78 °C and aldehyde 10 (218 mg in 500 μL dry ether, 0.4 mmol) was added dropwise to the reaction mixture via a microliter syringe at −78 °C. The cold bath was removed and the mixture was allowed to warm to ambient temperature and stirred for 24 h. The reaction was cooled to 0 °C. To the 0 °C mixture was added saturated NaHCO3 (1.0 mL) followed by slow addition of 30% H2O2 (2.0 mL). The reaction was stirred vigorously for ~5 h at room temperature. Brine (5 mL) was added, the organic layer was separated and the aqueous layer was extracted with Et2O (3 × 5 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. Purification of the crude product was performed by flash column chromatography (silica gel neutralized with 1% Et3N in hexane; gradient elution, hexane:Et2O = 100:1 to 10:1), which provided stereoheptad 33 (280 mg, 78% yield; ds > 15:1) as a colorless oil, contaminated with small amounts of isopinocamphenol. This intermediate was used directly in the following reaction without future purification: 1H NMR (400 MHz, CDCl3) δ 6.06 (dd, J = 19.2, 6.8 Hz, 1H), 5.95 (d, J = 19.2 Hz, 1H), 3.69–3.81 (m, 4H), 3.49 (bs, 1H), 3.43 (dd, J = 10.0, 8.4 Hz, 1H), 2.22–2.30 (m, 1H), 1.98–2.05 (m, 1H), 1.76–1.88 (m, 2H), 1.41–1.58 (m, 6H), 1.25–1.34 (m, 6H), 0.58–0.99 (m, 54H), 0.134 (s, 3H), 0.13 (s, 3H), 0.09 (s, 3H), 0.07 (s, 3H), 0.044 (s, 3H), 0.040 (s, 3H).
To a mixture of iodide 11a13 (12 mg, 60 μmol), vinyl stannane 33 (45 mg, 50 μmol), tris-(dibenzylideneacetone)-dipalladium(0) (2.3 mg, 2.5 mmol) and triphenylarsine (6 mg, 20 μmol) was added NMP (1.0 mL, freshly degassed by several freeze-thaw cycles under Ar). The resultant suspension was protected from light, warmed to 60 °C and stirred for 12 h. Then the reaction mixture (brown) was cooled to ambient temperature and partitioned between EtOAc and H2O. The aqueous layer was extracted with EtOAc (3 × 3 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. Purification of the crude product was performed by flash column chromatography (gradient elution, hexane:EtOAc = 20:1 to 1:2), which provided 8 (21 mg, 61%) as a colorless oil: [α]D 29.2 = −12.5° (c 0.88, CHCl3); H NMR (400 MHz, CDCl3) δ 7.41 (dd, J = 15.2, 11.2 Hz, 1H), 6.50 (dd, J = 11.6, 11.6 Hz, 1H), 6.19 (dd, J = 15.6, 8.0 Hz, 1H), 5.53 (d, J = 11.2 Hz, 1H), 5.39 (bs, 1H), 5.27 (bs, 1H), 3.87 (d, J = 8.8 Hz, 1H), 3.79 (dd, J = 6.8, 2.0 Hz, 1H), 3.73 (dd, J = 4.8, 4.4 Hz, 1H), 3.69 (dd, J = 10.0, 5.2 Hz, 1H), 3.61 (s, 1H), 3.43 (dd, J = 10.0, 8.0 Hz, 1H), 2.39–2.48 (m, 1H), 1.97–2.05 (m, 1H), 1.76–1.87 (m, 2H), 1.02 (d, J = 7.2 Hz, 3H), 1.00 (d, J = 6.8 Hz, 3H), 0.95 (d, J = 7.2 Hz, 3H), 0.92 (d, J = 6.8 Hz, 3H), 0.915 (s, 9H), 0.903 (s, 9H), 0.896 (s, 9H), 0.15 (s, 3H), 0.14 (s, 3H), 0.09 (s, 3H), 0.08 (s, 3H), 0.044 (s, 3H), 0.041 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 168.9, 148.1, 143.3, 126.5, 117.8, 81.4, 75.8, 75.3, 65.1, 42.2, 41.4, 41.0, 35.1, 26.6, 26.5, 26.3, 19.0, 18.7, 18.6, 16.9, 14.9, 13.0, 12.3, −2.7, −2.9, −3.1, −3.8, −5.0; IR (neat) 3501, 2958, 2931, 2859, 1668, 1593, 1464, 1389, 1323, 1257, 1086, 1027, 1004, 836, 775 cm−1; HRMS (ESI) m/z for C35H74NO5Si3 [M+H]+ calcd 672.4875, found 672.4869.
(2Z,4E,6S,7S,8R,9R,10S,11R,12R)-9,11,13-tris((tert-butyldimethylsilyl)oxy)-7-hydroxy-2,6,8,10,12-pentamethyltrideca-2,4-dienamide (9)
To a mixture of iodide 11b6a (13 mg, 60 μmol), vinyl stannane 33 (45 mg, 50 μmol), tris-(dibenzylideneacetone)-dipalladium(0) (2.3 mg, 2.5 μmol) and triphenylarsine (6 mg, 20 μmol) was added NMP (1.0 mL, freshly degassed by several freeze-thaw cycles under Ar). The resultant suspension was protected from light, warmed to 60 °C and stirred for 12 h. The reaction mixture (brown) was cooled to ambient temperature and partitioned between EtOAc and H2O. The aqueous layer was extracted with EtOAc (3 × 3 mL). The combined organic extracts were dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. Purification of the crude product was performed by flash column chromatography (gradient elution, hexane:EtOAc = 20:1 to 1:2), which provided 9 (23 mg, 61%) as a colorless oil: [α]D29.2 = +11.2° (c 0.82, CHCl3); 1H NMR (400 MHz, CDCl3) δ 6.74 (dd, J = 15.2, 10.8 Hz, 1H), 6.24 (d, J = 10.8 Hz, 1H), 5.93 (dd, J = 15.2, 8.0 Hz, 1H), 5.72 (bs, 1H), 5.54 (bs, 1H), 3.83 (d, J = 9.2 Hz, 1H), 3.79 (dd, J = 6.8, 2.0 Hz, 1H), 3.71 (dd, J = 4.8, 4.8 Hz, 1H), 3.69 (dd, J = 10.0, 4.8 Hz, 1H), 3.67 (s, 1H), 3.42 (dd, J = 10.0, 8.0 Hz, 1H), 2.29–2.38 (m, 1H), 1.97–2.05 (m, 1H), 1.95 (d, J = 0.8 Hz, 3H), 1.73–1.86 (m, 2H), 1.01 (d, J = 7.2 Hz, 3H), 0.95 (d, J = 6.8 Hz, 3H), 0.94 (d, J = 7.2 Hz, 3H), 0.92 (d, J = 6.8 Hz, 3H), 0.91 (s, 9H), 0.90 (s, 9H), 0.89 (s, 9H), 0.14 (s, 3H), 0.13 (s, 3H), 0.08 (s, 3H), 0.07 (s, 3H), 0.042 (s, 3H), 0.038 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 117.8, 143.2, 135.2, 128.8, 126.6, 81.3, 75.8, 75.3, 65.1, 42.2, 41.4, 41.0, 35.0, 26.6, 26.5, 26.3, 21.2, 19.0, 18.7, 18.6, 17.2, 14.9, 13.0, 12.3, −2.7, −2.9, −3.1, −3.8, −4.95, −4.96; IR (neat) 3368, 2958, 2931, 2859, 1662, 1599, 1464, 1386, 1257, 1086, 1030, 836, 775 cm−1; HRMS (ESI) m/z for C36H76NO5Si3 [M+H]+ calcd 686.5031, found 686.5027.
Supplementary Material
Acknowledgment
Financial support provided by the NIH (GM038436) and a predoctoral fellowship to M.C. from Eli Lilly is gratefully acknowledged.
Footnotes
Supporting Information Available. Details of stereochemical assignments and copies of 1H and 13C NMR spectra of new compounds are available via the internet at http://pubs.acs.org.
Reference
- 1.Sensi P, Margalith P, Timbal M. Farmaco Ed. Sci. 1959;14:146. [PubMed] [Google Scholar]; For a recent review, see Floss HG, Yu TW. Chem. Rev. 2005;105:621. doi: 10.1021/cr030112j.
- 2.Porter JR, Ge J, Lee J, Normant E, West K. Curr. Top. Med. Chem. 2009;9:1386. doi: 10.2174/156802609789895719. [DOI] [PubMed] [Google Scholar]
- 3.For total synthesis of rifamycin S, see; Nagaoka H, Rutsh W, Schmid G, Iio H, Johnson MR, Kishi Y. J. Am. Chem. Soc. 1980;102:7962.. Iio H, Nagaoka H, Kishi Y. J. Am. Chem. Soc. 1980;102:7965.. Nagaoka H, Kishi Y. Tetrahedron. 1981;37:3873.. Kishi Y. Pure Appl. Chem. 1981;53:1163.
- 4.For selected syntheses of the ansa chain of rifamycin S: Corey EJ, Hase T. Tetrahedron Lett. 1979;20:335.. Masamune S, Imperiali B, Garvey DS. J. Am. Chem. Soc. 1982;104:5528.. Hanessian S, Pougny J-R, Bossenkool IK. J. Am. Chem. Soc. 1982;104:6164.. Still WC, Barrish JC. J. Am. Chem. Soc. 1983;105:2487.. Fraser-Reid B, Magdzinski L, Molino B. J. Am. Chem. Soc. 1984;106:731.. Danishefsky SJ, Myles DC, Harvey DF. J. Am. Chem. Soc. 1987;109:862.. Roush WR, Palkowitz AD. J. Am. Chem. Soc. 1987;109:953.. Ziegler FE, Carn WT, Kneisly A, Stirchak EP, Wester RT. J. Am. Chem. Soc. 1988;110:5442.. Paterson I, McClure CK, Schumann RC. Tetrahedron Lett. 1989;30:1293.. Roush WR, Palkowitz AD, Ando K. J. Am. Chem. Soc. 1990;112:6348.. Miyashita M, Yoshihara K, Kawamine K, Hoshino M, Irie H. Tetrahedron Lett. 1993;34:6285.. Chenevert R, Rose YS. J. Org. Chem. 2000;65:1707. doi: 10.1021/jo991437w.. Harada T, Egusa T, Igarashi Y, Kinugasa M, Oku A. J. Org. Chem. 2002;67:7080. doi: 10.1021/jo025944g.
- 5.Matsuda S, Adachi K, Matsuo Y, Nukina M, Shizuri Y. J. Antibiot. 2009;62:519. doi: 10.1038/ja.2009.75. [DOI] [PubMed] [Google Scholar]
- 6.For total syntheses of saliniketals A and B, two closely related analogs of salinisporamycin, see: Paterson I, Razzak M, Anderson EA. Org. Lett. 2008;10:3295. doi: 10.1021/ol801148d.. Yadav JS, Hossain SS, Madhu M, Mohapatra DK. J. Org. Chem. 2009;74:8822. doi: 10.1021/jo901913h.. Liu J, De Brabander JK. J. Am. Chem. Soc. 2009;131:12562. doi: 10.1021/ja9061757.
- 7.Rateb ME, Houssen WE, Arnold M, Abdelrahman MH, Deng H, Harrison WTA, Okoro CK, Asenjo JA, Andrews BA, Ferguson G, Bull AT, Goodfellow M, Ebel R, Jaspars M. J. Nat. Prod. 2011;74:1491. doi: 10.1021/np200320u. [DOI] [PubMed] [Google Scholar]
- 8.(a) Hoffmann RW. Angew. Chem., Int. Ed. Engl. 1987;26:489. [Google Scholar]; (b) Hoffmann RW, Dahmann G, Andersen MW. Synthesis. 1994;629 [Google Scholar]
- 9.(a) Roush WR, Halterman RL. J. Am. Chem. Soc. 1986;108:294. [Google Scholar]; (b) Roush WR, Ando K, Powers DB, Palkowitz AD, Halterman RL. J. Am. Chem. Soc. 1990;112:6339. [Google Scholar]
- 10.Masamune S, Choy W, Petersen JS, Sita LR. Angew. Chem., Int. Ed. Engl. 1985;24:1. [Google Scholar]
- 11.Chen M, Roush WR. J. Am. Chem. Soc. 2012;134:3925. doi: 10.1021/ja300472a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Roush WR. J. Am. Chem. Soc. 2011;133:5744. doi: 10.1021/ja2010187. [DOI] [PMC free article] [PubMed] [Google Scholar]; For selected synthetic applications of reagent (S)-E-4, see: Chen M, Roush WR. Org. Lett. 2012;14:426. doi: 10.1021/ol203161u.. Chen M, Roush WR. Org. Lett. 2012;14:1880. doi: 10.1021/ol300476f.. For a related 9-BBN derived reagent that gives (Z)-vinylstannane products, see: Yamamoto Y, Yatagai H, Maruyama K. J. Am. Chem. Soc. 1981;103:3229.. Yamamoto Y, Maruyama K, Komatsu T, Ito W. J. Org. Chem. 1986;51:886. doi: 10.1021/ja00284a048.
- 13.(a) Ma S, Lu X, Li Z. J. Org. Chem. 1992;57:709. [Google Scholar]; (b) Buynak JD, Vogeti L, Chen H. Org. Lett. 2001;3:2953. doi: 10.1021/ol016142v. [DOI] [PubMed] [Google Scholar]
- 14.(a) Choy N, Shin YS, Nguyen PQ, Curran DP, Balachandran R, Madiraju C, Day BW. Org. Lett. 2002;4:4443. doi: 10.1021/ol026942l. [DOI] [PubMed] [Google Scholar]; (b) Izgu EC, Burns AC, Hoye TR. Org. Lett. 2011;13:703. doi: 10.1021/ol102936z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Brown HC, Bhat KS. J. Am. Chem. Soc. 1986;108:293. doi: 10.1021/ja00279a042. [DOI] [PubMed] [Google Scholar]; (b) Ramachandran PV, Srivastava A, Hazra D. Org. Lett. 2007;9:157. doi: 10.1021/ol062737k. [DOI] [PubMed] [Google Scholar]; (c) Waetzig JD, Hanson PR. Org. Lett. 2008;10:109. doi: 10.1021/ol7025944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Guo J, Duffy KJ, Stevens KL, Dalko PI, Roth RM, Hayward MM, Kishi Y. Angew. Chem., Int. Ed. 1998;37:187. [Google Scholar]; (b) Zhou W, Jimenez M, Jung W-H, Camarco DP, Balachandran R, Vogt A, Day BW, Curran DP. J. Am. Chem. Soc. 2010;132:9175. doi: 10.1021/ja103537u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Cherest M, Felkin H, Prudent N. Tetrahedron Lett. 1968;18:2199. [Google Scholar]; (b) Anh NT, Eisenstein O. Nouv. J. Chim. 1977;1:61. [Google Scholar]
- 18.Roush WR. J. Org. Chem. 1991;56:4151. [Google Scholar]
- 19.For selected examples, see: Roush WR, Palkowitz AD, Palmer MJ. J. Org. Chem. 1987;52:316.. Brown HC, Bhat KS, Randad RS. J. Org. Chem. 1989;54:1570.. Kim H, Ho S, Leighton JLJ. Am. Chem. Soc. 2011;133:6517. doi: 10.1021/ja200712f.. Roush WR. In: Comprehensive Organic Synthesis. Trost BM, editor. Vol. 2. Pergamon Press; Oxford: 1991. p. 1.
- 20.The conformations of aldehydes 13, 23, 24 and 25 in transition states TS-1 through TS-5 are drawn in such a way to minimize gauche pentane interactions. See, for example, Hoffman RW. Angew. Chem. Int. Ed. Engl. 1992;31:1124.
- 21.(a) Stille JK. Angew. Chem., Int. Ed. Engl. 1986;25:508. [Google Scholar]; (b) Farina V, Krishnamurthy V, Scott WJ. Org. React. 1997;50:1. [Google Scholar]
- 22.The spectroscopic and physical properties (e.g., 1H NMR, 13C NMR, IR, mass spectrum and/or C,H analysis) of all new compounds were fully consistent with the assigned structures. Yields refer to chromatographically and spectroscopically homogeneous materials (unless noted otherwise). Experimental procedures and tabulated spectroscopic data for other new compounds are provided in the Supporting Information
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.