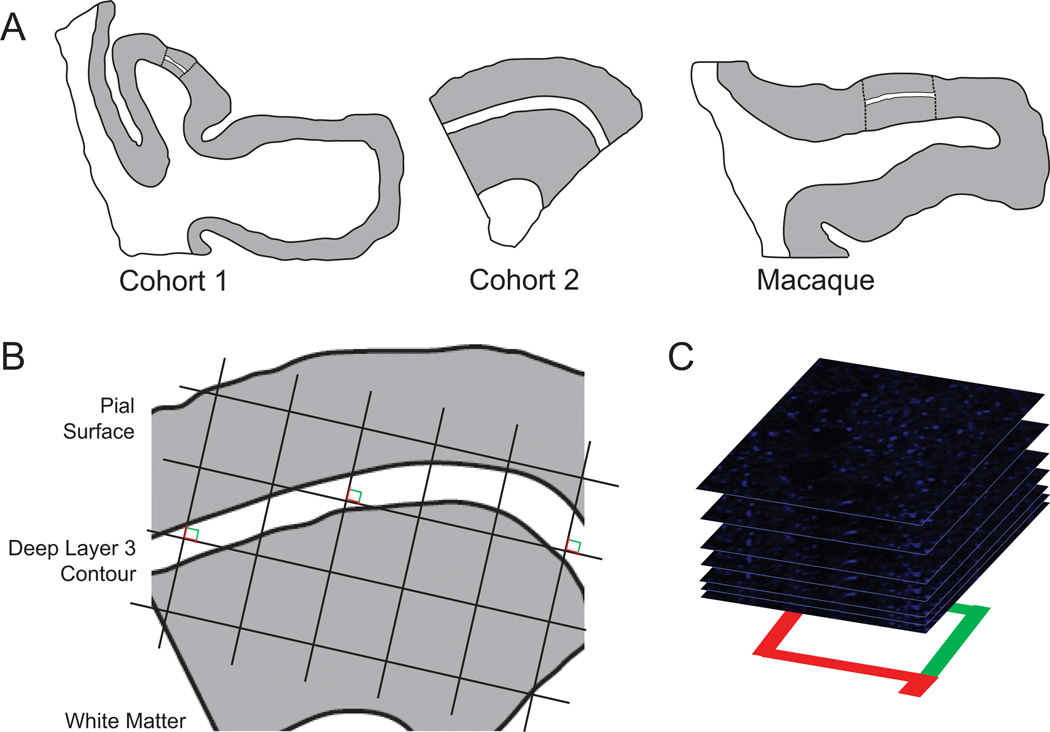
Figure 1.
Sampling of glutamate decarboxylase 65-immunoreactive boutons in primary auditory cortex deep layer 3. (A) Illustration of delineation of primary auditory cortex deep layer 3 on sections containing auditory cortex for human and antipsychotic exposed macaque cohorts. (Left) Cohort 1: Previously, every tenth section with a random start was selected from superior temporal gyrus blocks and processed for Nissl staining, and primary auditory cortex (Brodmann area 41) was identified using cytoarchitectonic criteria (42). Three Nissl-stained sections in which primary auditory cortex was cut perpendicular to the pial surface were selected for each subject, and sections adjacent to or nearby these sections were chosen for immunohistochemistry. (Middle) Cohort 2: Four primary auditory cortex blocks per subject were selected using a systematic uniformly random sampling scheme (90), designed to sample four primary auditory cortex blocks from each subject with equal probability. The central section of each block was stained for Nissl substance and used to delineate the cortical layer boundaries. From each selected block, one section adjacent to or nearby the center Nissl section was used in the present study. (Right) Antipsychotic-exposed macaques: Left hemisphere coronal superior temporal gyrus sections were generated similar to cohort 1 sections. Cytoarchitectonic criteria were used to identify primary auditory cortex, and three primary auditory cortex containing sections from each animal were selected for immunohistochemistry (43,44). For each human and monkey subject, the borders of layers 2/3 and 3/4 were identified on each of the adjacent Nissl-stained sections to determine the total layer 3 area for each subject. A contour outline (white) of the deepest one third of layer 3 was drawn in Stereo Investigator (MicroBright-Field Inc., Colchester, Vermont). The contours were aligned with the glutamate decarboxylase 65-labeled tissue sections using pial surface fiduciaries traced from the Nissl-stained sections. (B) A sampling grid was generated in Stereo Investigator to generate 12 to 14 sampling sites for each human subject and approximately 20 sites for each nonhuman primate subject. The grid size was determined based on the total deep layer 3 area across all tissue sections for a subject and the desired number of sampling sites per subject (12 for cohort 1 and 14 for cohort 2). The grid was then randomly rotated over the contour, and a sampling site was marked at every intersection between the grid and the deep layer 3 contour (shown as counting frames for the purpose of illustration; see Supplemental Methods in Supplement 1 for description of the associated point rule used in this study). (C) At each sampling site, a 10-µm thick stack of 46 image planes, each separated by .22 µm, was collected using spinning disk confocal microscopy.